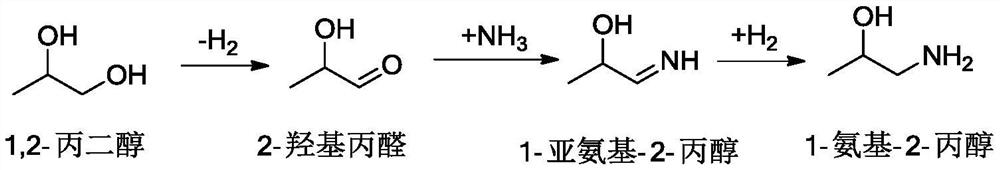
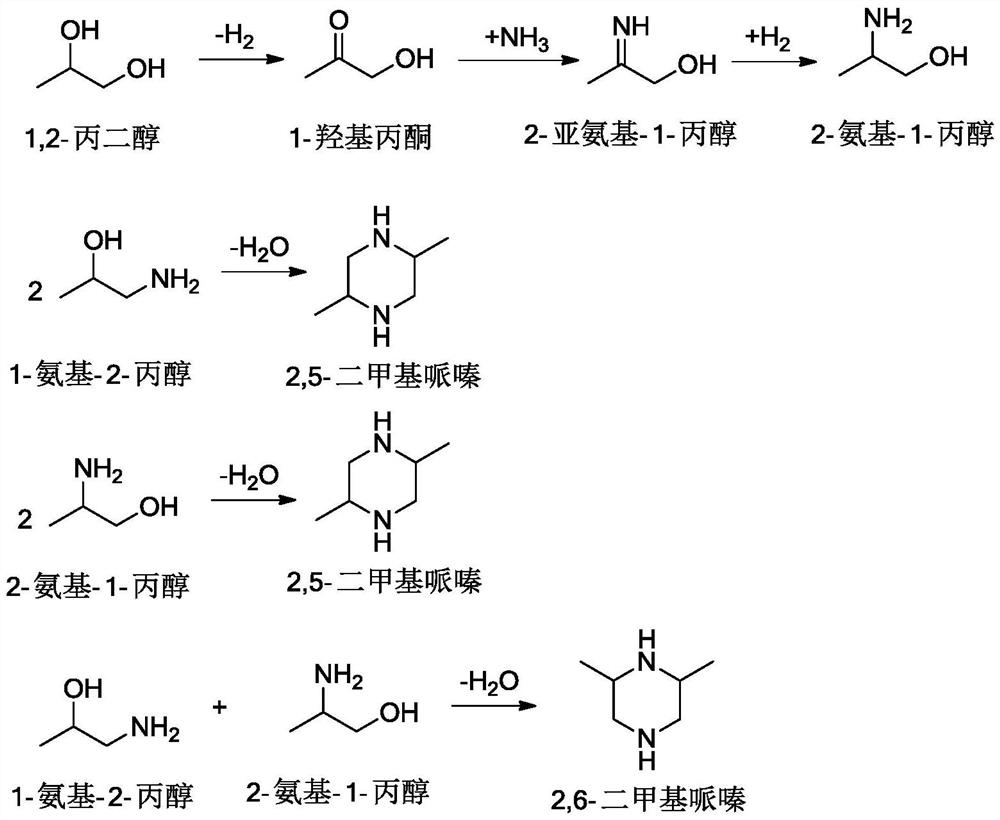
Method for preparing monoisopropanolamine
A technology of isopropanolamine and propylene glycol, which is applied in the field of preparation of isopropanolamine, can solve the problems of low conversion rate of raw materials, harsh reaction conditions, and low yield of products, and achieve the increase of alkaline sites, decrease of acidity, and reduction of dehydration The effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] (1) γ-Al 2 o 3 carrier modification
[0067] Using equal volume impregnation method, 300g γ-Al 2 o 3 Add it into 300ml aqueous solution containing 9.0g of tetrahydropyrrole, and after adsorption equilibrium, dry at 120°C for 5h, and roast at 300°C for 4h to obtain a modified γ-Al2O3 carrier.
[0068] (2) 5%CuO-5%PdO-0.5%Bi 2 o 3 -0.5%In 2 o 3 / Preparation of modified γ-Al2O3 catalyst
[0069] Using the equal volume impregnation method, the 100g modified γ-Al2O3 support obtained in step (1) was added to the carrier containing 15.2g Cu(NO 3 ) 2 ·3H 2 O, 9.41gPd(NO 3 ) 2 2H 2 O, 1.04g Bi(NO 3 ) 3 ·5H 2 O and 1.15g In(NO 3 ) 3 ·H 2 In 100ml aqueous solution of O, after adsorption equilibrium, dry at 130°C for 4h, and roast at 400°C for 5h to get 5%CuO-5%PdO-0.5%Bi 2 o 3 -0.5%In 2 o 3 / Modified γ-Al2O3 catalyst.
[0070] (3) 5%NiO-2%V 2 o 5 -0.4%Y 2 o 3 / Preparation of modified γ-Al2O3 catalyst
[0071] Using the equal volume impregnation metho...
Embodiment 2
[0121] (1) γ-Al 2 o 3 carrier modification
[0122] Using equal volume impregnation method, 300g γ-Al 2 o 3 Add it into 300ml aqueous solution containing 21.0g of tetrahydropyrrole, and after adsorption equilibrium, dry at 140°C for 4h, and roast at 400°C for 5h to obtain a modified γ-Al2O3 carrier.
[0123] (2) 6%CuO-4%PdO-3%Bi 2 o 3 -0.2%In 2 o 3 / Preparation of modified γ-Al2O3 catalyst
[0124] Using the equal volume impregnation method, the 100g modified γ-Al2O3 support obtained in step (1) was added to the carrier containing 18.2g Cu(NO 3 ) 2 ·3H 2 O, 7.53gPd(NO 3 ) 2 2H 2 O, 6.25g Bi(NO 3 ) 3 ·5H 2 O and 0.46g In(NO 3 ) 3 ·H 2 In 100ml aqueous solution of O, after adsorption equilibrium, dry at 140°C for 6h, and roast at 350°C for 4h to get 6%CuO-4%PdO-3%Bi 2 o 3 -0.2%In 2 o 3 / Modified γ-Al2O3 catalyst.
[0125] (3) 4%NiO-1%V 2 o 5 -0.1%Y 2 o 3 / Preparation of modified γ-Al2O3 catalyst
[0126] Using the equal volume impregnation method, ...
Embodiment 3
[0134] (1) γ-Al 2 o 3 carrier modification
[0135] Using equal volume impregnation method, 300g γ-Al 2 o 3 Add it into 300ml aqueous solution containing 15.0g piperidine, after adsorption equilibrium, dry at 130°C for 6h, and roast at 500°C for 6h to obtain modified γ-Al2O3 carrier.
[0136] (2) 7%CuO-3%PdO-2.5%Bi 2 o 3 -0.15%In 2 o 3 / Preparation of modified γ-Al2O3 catalyst
[0137] Using the equal volume impregnation method, add the 100g modified γ-Al2O3 carrier obtained in step (1) into the carrier containing 21.3g Cu(NO 3 ) 2 ·3H 2 O, 5.65gPd(NO 3 ) 2 2H 2 O, 5.21g Bi(NO 3 ) 3 ·5H 2 O and 0.34g In(NO 3 ) 3 ·H 2 In 100ml aqueous solution of O, after adsorption equilibrium, dry at 120°C for 3h, and roast at 300°C for 6h to obtain 7%CuO-3%PdO-2.5%Bi 2 o 3 -0.15%In 2 o 3 / Modified γ-Al2O3 catalyst.
[0138] (3) 3%NiO-3%V 2 o 5 -0.5%Y 2 o 3 / Preparation of modified γ-Al2O3 catalyst
[0139] Using the equal volume impregnation method, the 100g mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com