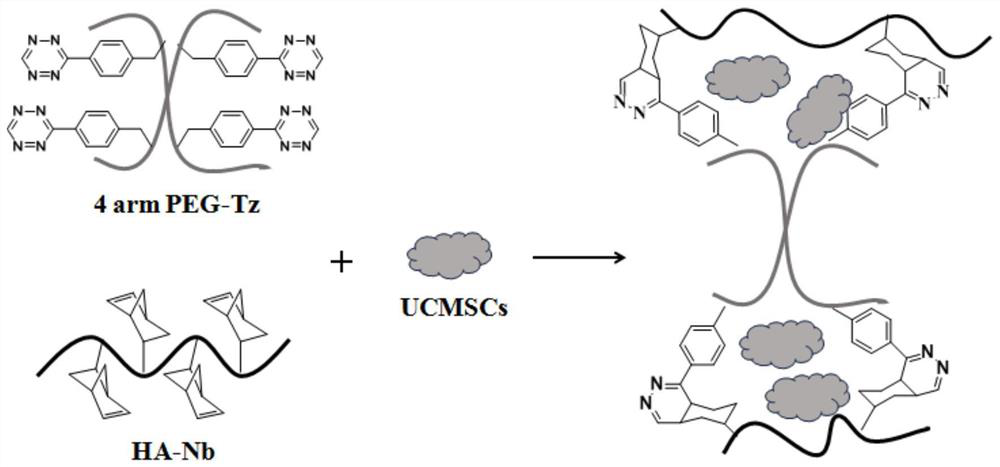
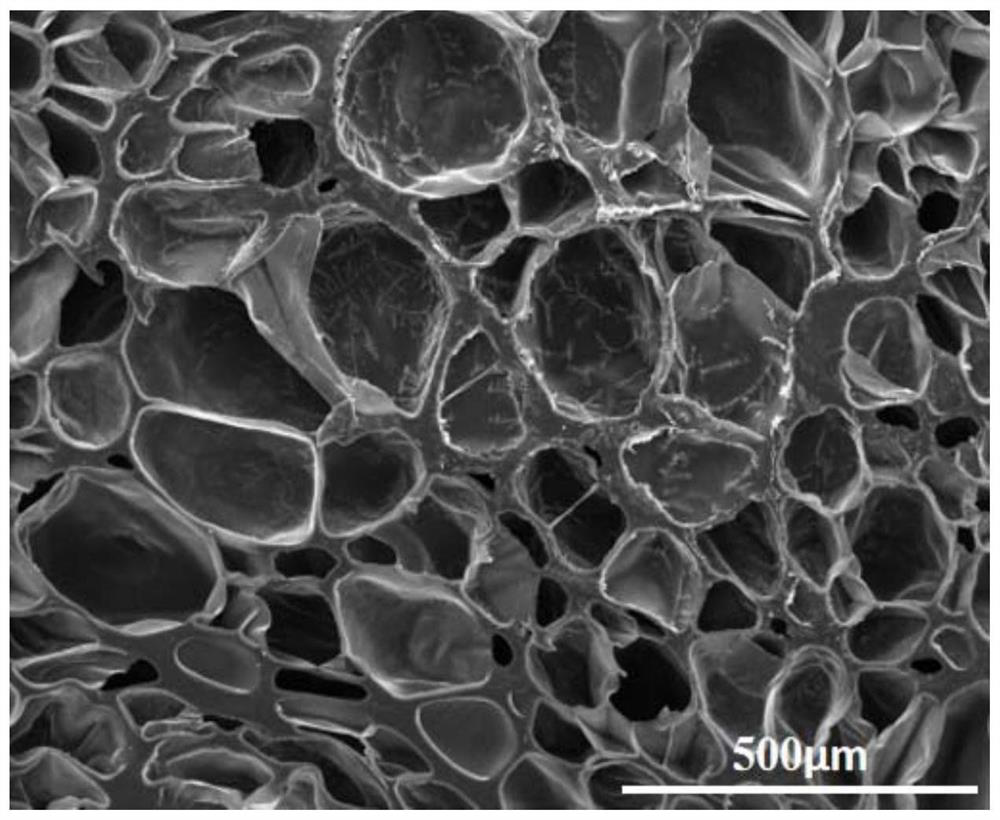
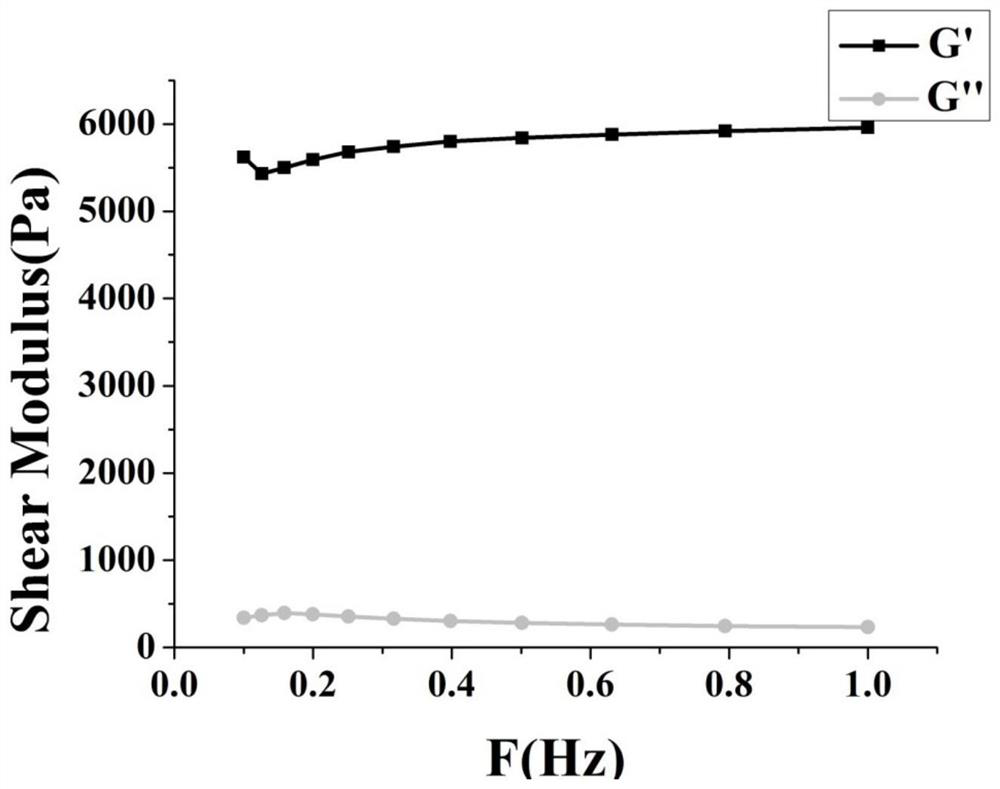
Hydrogel capable of being rapidly cured based on reverse Diels-Alder reaction as well as preparation method and application thereof
A hydrogel and reaction technology, applied in biochemical equipment and methods, 3D culture, microorganisms, etc., can solve problems such as cell damage, and achieve the effects of low toxicity, good biocompatibility, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] In some embodiments, the preparation method includes: mixing four-armed polyethylene glycol and a condensing agent in a first solvent to obtain a first mixed system, and then reacting the first mixed system at 0-8°C for 10-30 minutes ; Then add 3-(4-benzylamino)-1,2,4,5-tetrazine to form a second mixed system, and then react the second mixed system at 15~30°C for 10~20h to obtain tetrazine-modified Four-armed polyethylene glycol; preferably, the first solvent includes dichloromethane.
[0054] Further, the condensing agent includes benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate and N,N-diisopropylethylamine.
[0055] Further, the molar ratio of the condensing agent to four-arm polyethylene glycol is 1-3:1.
[0056] Further, the molar ratio of the four-arm polyethylene glycol to 3-(4-benzylamino)-1,2,4,5-tetrazine is 1:1-3.
[0057] Further, the preparation method further includes: after the reaction of the second mixed system is completed, addi...
Embodiment 1
[0070] Step 1: Add 4armPEG-COOH (M w =10000) was dissolved in dichloromethane, then added benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate and N,N-diisopropylethylamine, ice bath After activating for 15 minutes, add 3-(4-benzylamino)-1,2,4,5-tetrazine and react overnight.
[0071] Among them, the carboxyl group on 4armPEG-COOH, 3-(4-benzylamino)-1,2,4,5-tetrazine, 1H-benzotriazol-1-yloxytripyrrolidinylphosphonium hexafluorophosphate And the reaction molar ratio of N,N-diisopropylethylamine is 1:1.2:2.5:2.5.
[0072] After the reaction in step 1 is completed, precipitate in ether to obtain a pink precipitate, centrifuge at 7000rpm for 5 minutes to collect the precipitate, dissolve the above precipitate in water, purify it through a G15 Sephadex column, and finally freeze-dry to obtain a tetrazine-modified PEG, tetrazine-modified PEG. The PEG structural formula modified by oxazine is shown in formula (1):
[0073]
[0074] Step 2: sodium hyaluronate (...
Embodiment 2
[0084] Step 1: Add 4armPEG-COOH (M w =10000) was dissolved in dichloromethane, then added isobutyl chloroformate and N-methylmorpholine, activated at 4°C for 30min, and then added 3-(4-benzylamino)-1,2,4,5- Tetrazine, overnight reaction.
[0085] Among them, the carboxyl group on 4armPEG-COOH, 3-(4-benzylamino)-1,2,4,5-tetrazine, 1H-benzotriazol-1-yloxytripyrrolidinylphosphonium hexafluorophosphate And the reaction molar ratio of N,N-diisopropylethylamine is 1:2:3:3.
[0086] After the reaction in step 1, precipitate in ether to obtain a pink precipitate, centrifuge at 8000 rpm for 5 min to collect the precipitate, dissolve the above precipitate in water, purify it through a G-15 Sephadex column, and finally freeze-dry to obtain the tetrazine-modified tetrazine Arm polyethylene glycol, tetrazine-modified four-arm polyethylene glycol structural formula as shown in formula (1):
[0087]
[0088] Step 2: sodium hyaluronate (M w =36KDa) was dissolved in MES buffer solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com