Preparation method of cobalt-free positive electrode material
A positive electrode material and system technology, applied in the field of preparation of cobalt-free positive electrode materials, to achieve the effects of excellent phase change reversibility and structural stability, ease of dissolution, and large discharge specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The invention provides a method for preparing a cobalt-free positive electrode material, comprising the following steps:
[0031] (1) Mix nickel sulfate and manganese sulfate salt solution according to Ni:Mn stoichiometric ratio 0.75:0.25, then add nano-alumina according to Al:Ni stoichiometric ratio 0.005:1, ultrasonic; in nitrogen atmosphere, mix the above solution into the reaction kettle, and then add a mixed alkali solution of NaOH and ammonia water to adjust the pH to 11.3, and the reaction temperature to 50°C. After the reaction, wash, filter, and dry.
[0032] (2) Lithium hydroxide (LiOH·H 2 O) Mix evenly with the powder obtained in step (1), calcine at a constant temperature of 910° C. for 12 hours in an oxygen atmosphere, and cool naturally to obtain a cobalt-free positive electrode material.
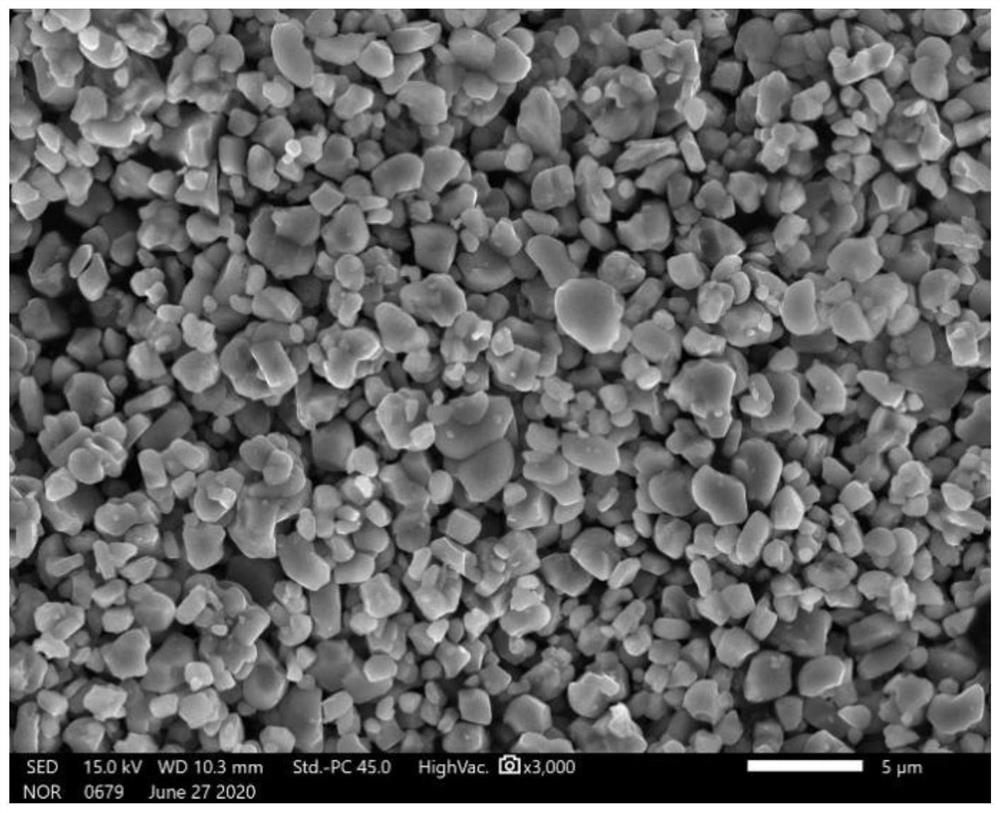
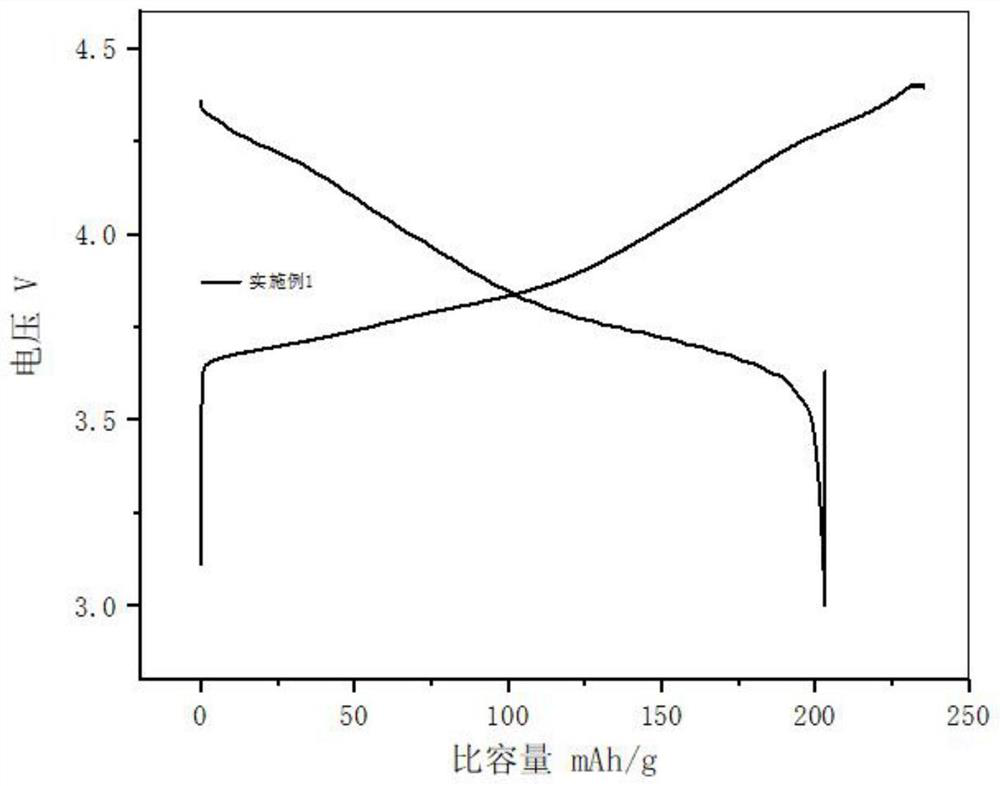
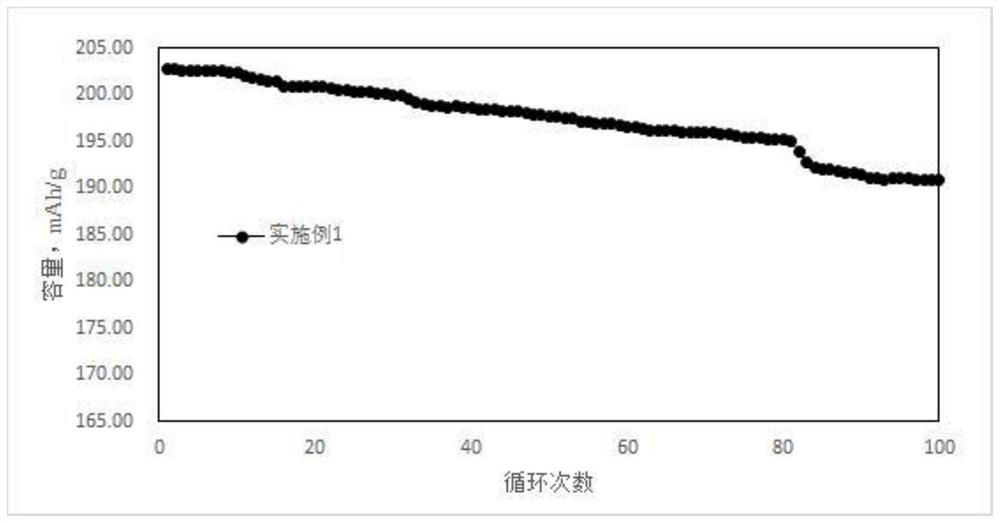
[0033] In conjunction with the accompanying drawings, the phase characterization and electrochemical performance of the cobalt-free positive electrode material prepare...
Embodiment 2
[0037] (1) Mix nickel nitrate and manganese nitrate salt solution by Ni:Mn stoichiometric ratio 0.60:0.40, then add nano-alumina and nano-magnesia by Al:Mg:Ni stoichiometric ratio 0.005:0.003:1, ultrasonic; In a nitrogen atmosphere, the above mixed solution was added to the reactor, and then a mixed alkali solution of KOH and ammonia was added to adjust the pH to 11.2, and the reaction temperature was 60°C. After the reaction, it was washed, filtered and dried.
[0038] (2) Lithium hydroxide (LiOH·H 2 O) Mix evenly with the powder obtained in step (1), calcinate at a constant temperature of 760° C. for 15 hours in an oxygen atmosphere, and cool naturally to obtain a cobalt-free positive electrode material.
[0039] The discharge specific capacity of the cobalt-free cathode material prepared in Example 2 is 183.5mAh / g, and the capacity retention rate remains at 92.33% after 100 cycles.
Embodiment 3
[0041] (1) Mix nickel chloride and manganese chloride salt solution by Ni:Mn stoichiometric ratio 0.70:0.30, then add nano-tungsten oxide by W:Ni stoichiometric ratio 0.008:1, ultrasonic; in nitrogen atmosphere, put The above-mentioned ion mixture is added to the reaction kettle, and then a mixed alkali solution of KOH and ammonia water is added to adjust the pH to 11.2, and the reaction temperature is 50°C. After the reaction is completed, it is washed, filtered, and dried.
[0042] (2) Lithium hydroxide (LiOH·H 2 O) Mix evenly with the powder obtained in step (1), calcinate at a constant temperature of 840° C. for 12 hours in an oxygen atmosphere, and cool naturally to obtain a cobalt-free positive electrode material.
[0043] The discharge specific capacity of the cobalt-free cathode material prepared in Example 3 is 194.3mAh / g, and the capacity retention rate remains at 94.28% after 100 cycles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com