Method for detecting content of citrulline in citrulline raw material
A detection method and citrulline technology, applied in the field of biological analysis, can solve the problems of affecting the accuracy of quantitative analysis of citrulline, damage to the service life of chromatographic columns, and expensive derivatization reagents, so as to reduce the economic burden of enterprises and shorten the detection time. , The effect of reducing the error rate of experimental operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The equipment, reagents and detection conditions used in this embodiment are as follows:
[0062] High performance liquid chromatography: Thermo Fisher Dionex Ultimate 3000; VWD UV detector;
[0063] Chromatographic column: Thermo Fisher Syncronis C18 (5μm, 4.6×250mm);
[0064] Column temperature: 30°C;
[0065] Wavelength: 190nm;
[0066] Flow rate: 0.6mL / min;
[0067] Injection volume: 10μL;
[0068] Mobile phase: Mobile phase A is a mixed solution of 0.05mol / L dipotassium hydrogen phosphate and 0.005mol / L sodium decanesulfonate, the pH value is 2.3, and mobile phase B is absolute ethanol;
[0069] Mobile phase volume ratio: 1000:70.
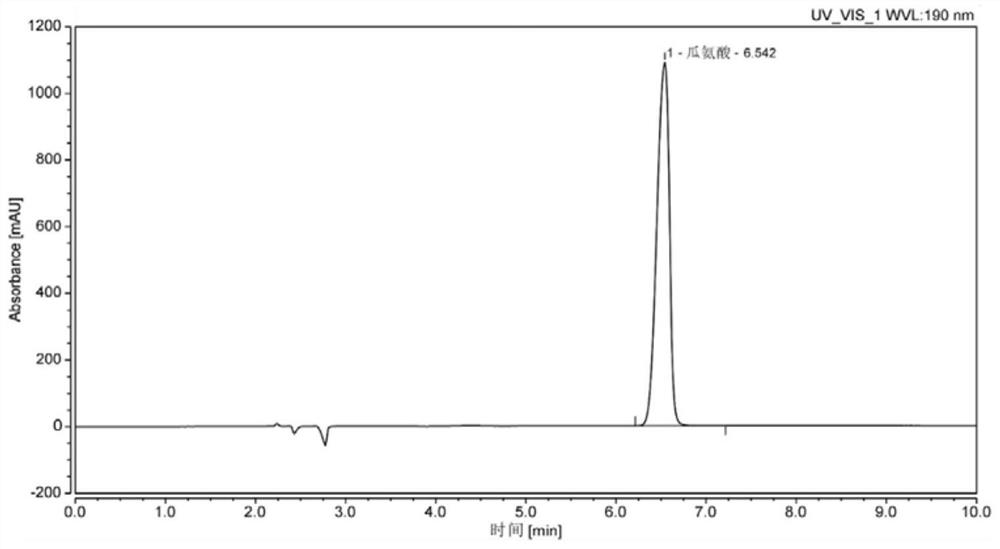
[0070] The specific operation steps for the determination of the content of citrulline in the reference substance:
[0071] (1) Reference substance solution preparation
[0072] Citrulline control stock solution: Accurately weigh an appropriate amount of citrulline reference substance, and dilute with deionized water to obtain a ...
Embodiment 2
[0076] The equipment, reagents and detection conditions used in this embodiment are as follows:
[0077] High performance liquid chromatography: Thermo Fisher Dionex Ultimate 3000; VWD UV detector;
[0078] Chromatographic column: Thermo Fisher Syncronis C18 (5μm, 4.6×250mm);
[0079] Column temperature: 30°C;
[0080] Wavelength: 190nm;
[0081] Flow rate: 0.6mL / min;
[0082] Injection volume: 10μL;
[0083] Mobile phase: Mobile phase A is a mixed solution of 0.05mol / L dipotassium hydrogen phosphate and 0.005mol / L sodium decanesulfonate, the pH value is 2.3, and mobile phase B is absolute ethanol;
[0084] Mobile phase volume ratio: 1000:70.
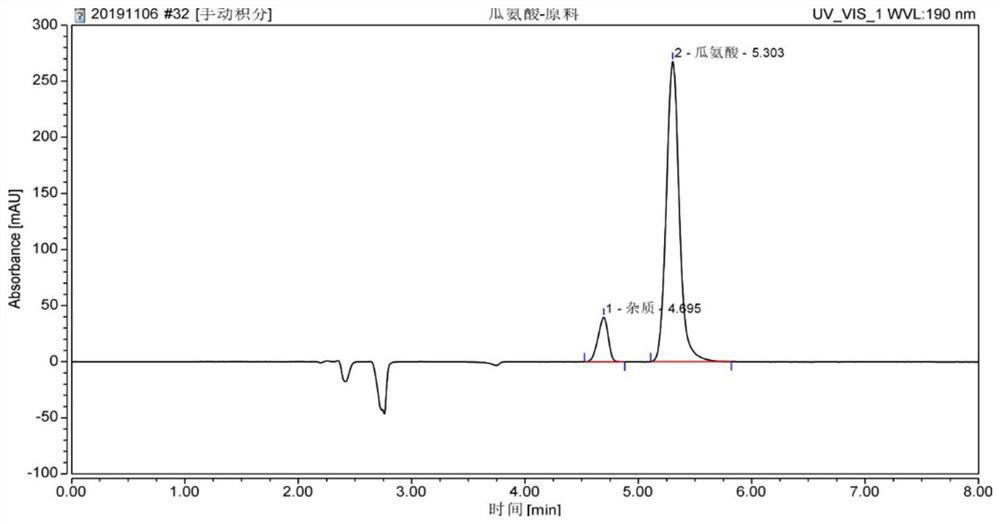
[0085] Determination of citrulline content in raw materials Concrete steps:
[0086] (1) Take 0.1g of citrulline raw material (the purity of citrulline is known to be 98.11%) and place it in a 100mL volumetric flask, use deionized water to set the volume to the mark, and shake well to obtain citrulline with a concentration of 1g / L...
Embodiment 3
[0090] The equipment, reagents and detection conditions used in this embodiment are as follows:
[0091] High performance liquid chromatography: Thermo Fisher Dionex Ultimate 3000; VWD UV detector;
[0092] Chromatographic column: Thermo Fisher Syncronis C18 (5μm, 4.6×250mm);
[0093] Column temperature: 30°C;
[0094] Wavelength: 190nm;
[0095] Flow rate: 0.6mL / min;
[0096] Injection volume: 10μL;
[0097] Mobile phase: Mobile phase A is a mixed solution of 0.05mol / L dipotassium hydrogen phosphate and 0.005mol / L sodium decanesulfonate, the pH value is 2.3, and mobile phase B is absolute ethanol;
[0098] Mobile phase volume ratio: 1000:70.
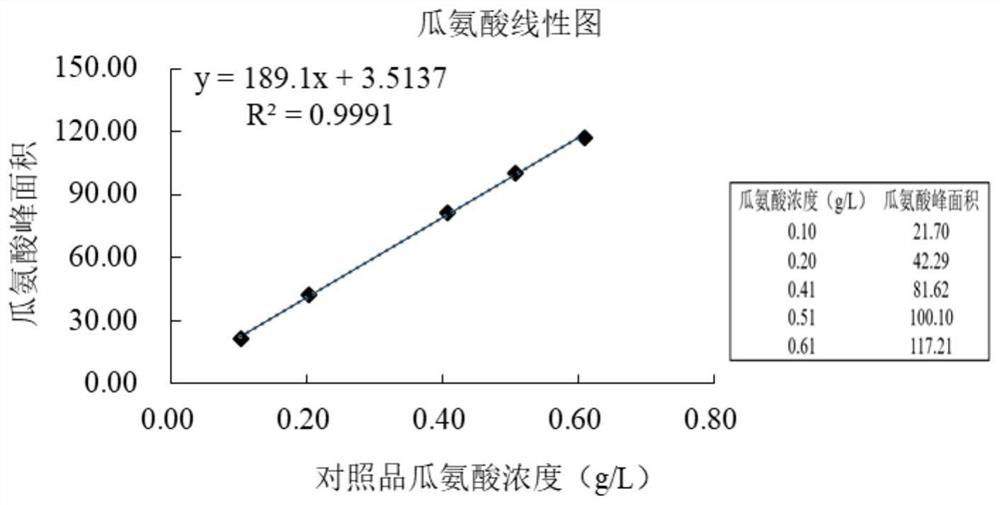
[0099] Citrulline linearity test specific steps:
[0100] (1) Accurately draw an appropriate amount of citrulline contrast stock solution prepared in Example 1 for 10 times respectively, place them in five 10ml volumetric flasks respectively, and dilute with deionized water to obtain a concentration of 0.10g / L, 0.20g / L, 0.41g / L, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Ph value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com