Preparation method for recombinant human antibody fusion protein
A fusion protein and human antibody technology, applied in the field of biomedicine, can solve the problems of cumbersome operation, low expression amount, and many processing opportunities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] 2. The preparation method of the present invention can express a high-purity recombinant human antibody fusion protein with a suitable glycosylation pattern.
[0100] 3. Compared with other eukaryotic expression systems, the DG44 expression system can efficiently express foreign genes with a high expression level;
[0101] 4. During the purification process, use affinity chromatography-anion-cation to obtain antibody protein with a purity greater than 99.8%, which is a simple, convenient, low-cost, and high-yield antibody fragment purification and preparation method;
[0102] 5. Since trace process-related impurities such as host cell protein (HCP) and host cell DNA (HCD) residues in biopharmaceuticals may have a certain impact on the safety and efficacy of biopharmaceuticals, precise HCP and HCD control is of great importance to The quality and safety of recombinant human antibody fusion proteins are of particular importance.
[0103] 6. The recombinant human antibody...
Embodiment 1
[0106] Example 1 Engineering Cell Line Construction of Recombinant Human Antibody Fusion Protein Gene
[0107] After the pCHO 1.0 vector was digested with AvrII (CCTAGG) and PacI (TTAATTAA), it was ligated with the recombinant human antibody fusion protein gene fragment, and the ligated product was transformed into DH5α E. coli competent cells, and spread on LB agar culture plates containing ampicillin resistance On the plate, through resistance screening, a single colony transformant was obtained on the plate. The recombinant plasmids were extracted after single-clonal colonies were selected and cultured, and the extracted recombinant plasmids were digested with AvrII / PacI double enzymes for DNA sequencing. The sequencing results of the recombinant plasmids were consistent with the expected sequence comparison.
[0108] The correct recombinant plasmid Pvu I of clone is digested and transformed into the CHO-DG44 host cell (purchased from Invitrogen Company) in the logarithmic ...
Embodiment 2
[0109] Example 2 Purification and Identification of Recombinant Human Antibody Fusion Protein
[0110] The engineered cell strain of Example 1 was induced by IPTG, and 1000 ml of culture supernatant was taken.
[0111] 1. Affinity chromatography
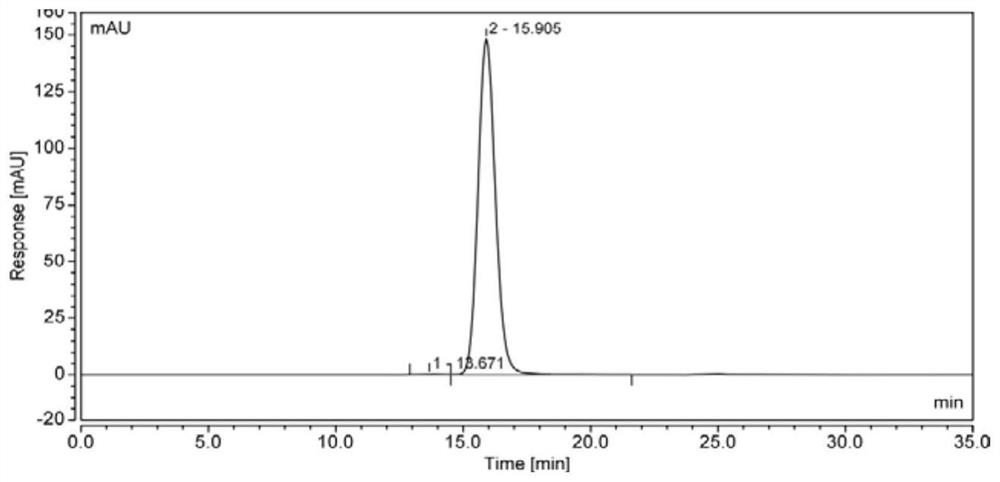
[0112] Get the filtrate and load it on the Protein ADiamond affinity chromatography column (filler comes from Bogelong Shanghai Biotechnology Co., Ltd.) equilibrated with "20mmol / L PB, 150mmol / L NaCl, pH 7 ± 0.5" for chromatography, use "50mmol / L CB, pH5±1” eluting, “50mmol / L CB, pH4.5±0.5” elution, detected by ELISA Book Example Recombinant Human Antibody Fusion Protein Peak, Collection of Flow Through.
[0113] 2. Low pH incubation and depth filtration
[0114] 2.1 Low pH incubation
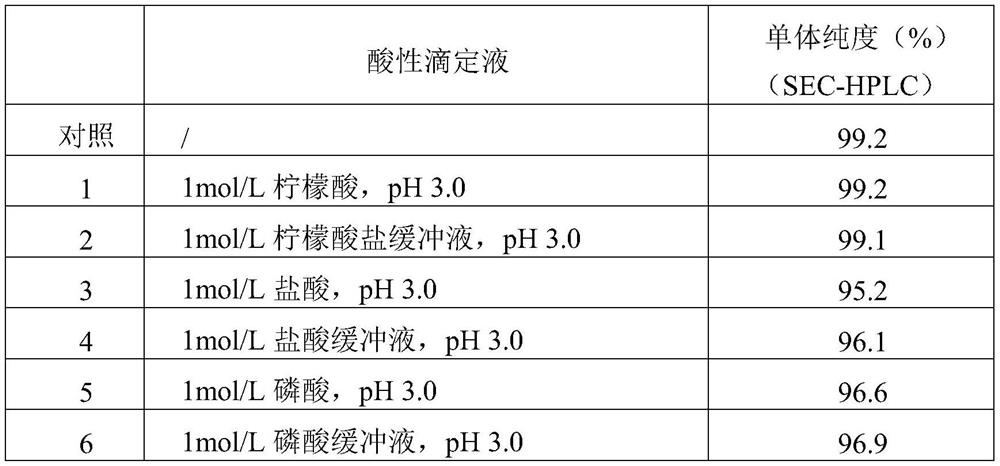
[0115] Selection of buffer: the affinity chromatography flow-through in step 1 is directly adjusted to pH 5.5 with 1mol / L Tris, and placed at 2-8°C for testing; the same batch of affinity chromatography flow-through in step 1 is used Adjust the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com