Method for preparing iron-chromium redox battery electrolyte
An electrolyte and battery technology, applied in the field of energy storage, can solve the problem of high cost and achieve the effect of digesting industrial waste and ensuring purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
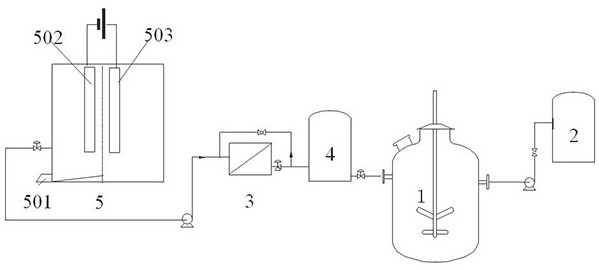
[0041] see figure 1 , the present embodiment proposes a system for preparing an electrolyte for an iron-chromium redox battery, comprising an electrolytic cell 5, a filter 3, and a reactor 1,
[0042] The electrolytic cell 5 includes a positive electrode chamber and a negative electrode chamber. The positive electrode chamber and the negative electrode chamber are separated by an ion-selective membrane. Both the positive electrode 503 and the negative electrode 502 are iron sheet electrodes, and the distance between the iron sheet electrodes and the bottom is 1 / 3 of the electrolytic cell. high;
[0043] The negative electrode chamber is connected to a filter 3 through a pipeline, and the filter 3 is connected to a buffer tank 4 through a pipeline, and the buffer tank 4 is connected to the reaction kettle 1 . Reactor 1 is connected with electrolyte storage tank 2 .
[0044] There is a slag outlet 501 at the bottom of the negative electrode chamber; the bottom of the negative ...
Embodiment 2
[0055] A method for preparing an electrolyte for an iron-chromium redox battery, using the system of Embodiment 1, including operations:
[0056] 1) The iron-containing material is dissolved in hydrochloric acid, the concentration of the hydrochloric acid is 0.2mol / L, an electrode is inserted in the solution, the electrode is set as the negative pole, and the current is electrolyzed; the iron-containing material is four Ferric oxide and red mud, the mass ratio of ferric oxide and red mud is 5:3, the mass ratio of red mud and ferric oxide total mass to 0.2mol / L hydrochloric acid is about 1:1,
[0057] The positive electrode solution for electrolysis is 0.2mol / L hydrochloric acid and 0.1mol / L sodium chloride;
[0058] The electrodes are iron electrodes. Using constant current electrolysis, set the current density to 0.2A / cm 2 , calculate the energization time according to the formula (1), so that the energization stops when the cell voltage is about -0.4V.
[0059] After the...
Embodiment 3
[0065] This embodiment proposes a method for preparing an electrolyte for an iron-chromium redox battery, using the system of Embodiment 1, including operations:
[0066] 1) The iron-containing material is dissolved in hydrochloric acid, the concentration of the hydrochloric acid is 0.2mol / L, an electrode is inserted in the solution, the electrode is set as the negative pole, and the current is electrolyzed; the iron-containing material is four Ferric oxide, the mass ratio of ferric oxide quality and 0.2mol / L hydrochloric acid is about 30:100,
[0067] The positive electrode solution for electrolysis is 0.2mol / L hydrochloric acid and 0.1mol / L sodium chloride;
[0068] The electrodes are iron electrodes. Using constant current electrolysis, set the current density to 0.2A / cm 2 , calculate the energization time according to the formula (1), so that the energization stops when the cell voltage is about -0.4V.
[0069] 2) Chromium chloride and hydrochloric acid are added in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com