Method for extracting rubidium chloride from rubidium-containing high-salt brine
A rubidium chloride and brine technology, applied in chemical instruments and methods, rubidium/cesium/francium compounds, inorganic chemistry, etc., can solve the problems of volume increase, increase the cost of recovering other salts in salt brine, etc., and reduce the cost of follow-up treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
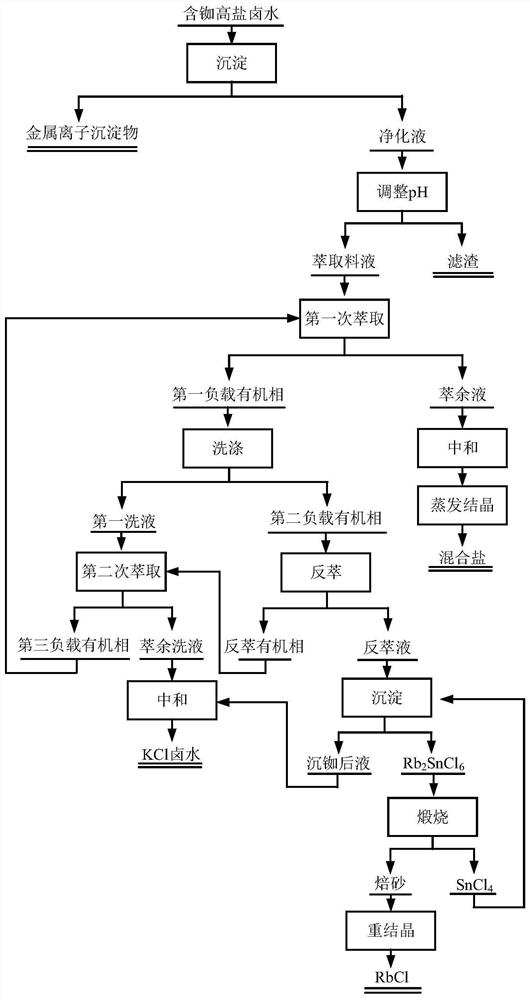
[0046] use figure 1 The process flow shown is to extract rubidium from a certain sintered mineral leach solution (ie high potassium salt brine containing rubidium), and its main metal components are shown in Table 1.
[0047] Table 1 Main metal components of sintered mineral leachate
[0048] Element Rb Cs K Na Cu Zn Content (g / L) 3.74 0.40 142.42 50.13 3.25 1.27
[0049] In the present embodiment, the method for extracting rubidium chloride from rubidium-containing high-salt brine, the steps are as follows:
[0050] S1: Add KOH to the rubidium-containing high-potassium salt brine (ie sintered mineral leachate), adjust the pH value of the rubidium-containing high-potassium salt brine to 10, let it stand for 2 hours, precipitate metal ions, and filter to remove the metal Ion precipitation to obtain a purified solution;
[0051] S2: adding KOH to the purification solution obtained in step S1, adjusting the pH value of the purification solut...
Embodiment 2
[0067] use figure 1 The process flow shown is to extract rubidium in a certain solid waste leach solution (ie high potassium salt brine containing rubidium), and its main metal components are shown in Table 5.
[0068] Table 5 Main metal components of solid waste leachate
[0069] Element Rb Cs K Na Cu Zn Pb Content (g / L) 2.74 0.005 102.21 23.16 1.90 10.02 1.20
[0070] In the present embodiment, the method for extracting rubidium chloride from rubidium-containing high-salt brine, the steps are as follows:
[0071] S1: Add KOH to the rubidium-containing high-potassium salt brine (i.e. solid waste leaching solution), adjust the pH value of the rubidium-containing high-potassium salt brine to 10, let it stand for 2 hours, precipitate metal ion precipitates, and filter to remove the metal Ion precipitation to obtain a purified solution;
[0072] S2: adding KOH to the purification solution obtained in step S1, adjusting the pH value of the...
Embodiment 3
[0088] use figure 1 The process flow shown is to extract rubidium in a simulated rubidium-containing high-potassium salt lake water (ie, rubidium-containing high-potassium salt brine), and its main metal components are shown in Table 9.
[0089] Table 9 The main metal components of simulated rubidium-containing high-potassium salt lake water
[0090] Element Rb Cs K Na Li Mg Content (g / L) 0.21 0.002 27.72 96.95 6.85 28.38
[0091] In the present embodiment, the method for extracting rubidium chloride from rubidium-containing high-salt brine, the steps are as follows:
[0092] S1: Add KOH to the rubidium-containing high-potassium salt brine (that is, the simulated rubidium-containing high-potassium salt lake water), adjust the pH value of the rubidium-containing high-potassium salt brine to 10, let stand for 2 hours, precipitate metal ions, and filter removing the metal ion precipitate to obtain a purified solution;
[0093] S2: adding KOH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com