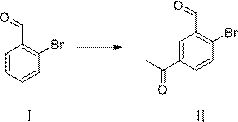
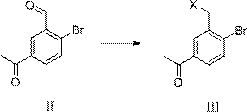
Synthesis method of 3-bromomethyl-4-bromoacetophenone
A technology of bromoacetophenone and synthesis method, which is applied in the preparation of carbonyl compounds, chemical instruments and methods, and preparation of carbonyl compounds by condensation, etc., can solve the problems of high price, high total cost, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of compound Ⅱ
[0048]At room temperature (20-25°C), dissolve 185.02g of o-bromobenzaldehyde in 185ml of acetamide, and add catalyst 154.66g of thionyl chloride dropwise at a temperature of 15-25°C. After dropping, keep warm at 20-25°C for reaction , monitor the reaction in the liquid phase, and the reaction is complete in about 10-13 hours. After the reaction is completed, add 300 g of ice water dropwise at a temperature of 0-30 ° C to destroy, add 600 ml of dichloroethane for extraction, and after liquid separation, wash the organic phase with 200 ml of water once. Liquid separation, dichloromethane was distilled off the organic phase under reduced pressure, ethyl acetate:petroleum ether=1:5 was added for recrystallization, and 190.5 g of the product was obtained as a light yellow solid with a content of 99.62% and a yield of 83.58%.
Embodiment 2
[0050] Synthesis of compound Ⅱ
[0051] At room temperature (20-25°C), dissolve 185.02g of o-bromobenzaldehyde and 70.89g of acetamide in 555ml of dichloromethane, and add catalyst 154.66g of thionyl chloride dropwise at a temperature of 15-25°C. , keep warm at 20-25°C, monitor the reaction in the liquid phase, and the reaction is complete in about 10-13 hours. After the reaction, control the temperature at 0-30°C and add 300g of ice water dropwise to destroy it. Liquid, dichloromethane was distilled off the organic phase under reduced pressure, ethyl acetate:petroleum ether=1:5 solvent was added for recrystallization, and 178.87g of the product was obtained as a light yellow solid with a content of 99.47% and a yield of 78.36%.
Embodiment 3
[0053] Synthesis of Compound Ⅲ-a
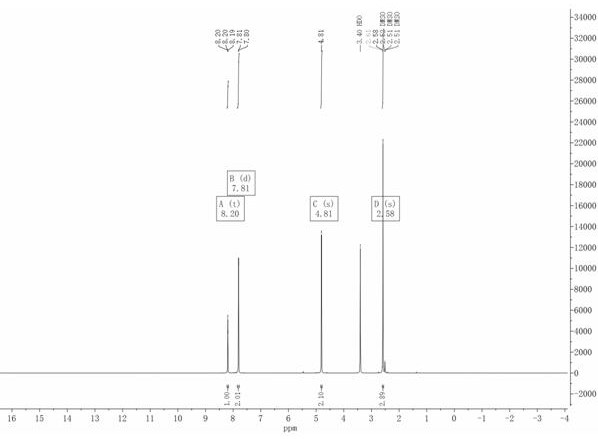
[0054] At room temperature (20-25°C), add 113.53g of compound II to 568ml of dichloromethane to dissolve, then add 65.18g of trimethylchlorosilane, 67.16g of 1,1,3,3 -Tetramethyldisiloxane, react at 30-40°C after addition, monitor the reaction in liquid phase, stop the reaction until the compound II is 0-3%, lower the temperature, add 300ml water at 0-20°C and stir for 30 minutes, separate the liquid, The organic phase was washed twice with water, 150ml each time, the solvent was recovered under reduced pressure, and the concentrated dry matter was recrystallized by adding ethyl acetate:petroleum ether=1:2.5 solvent, filtered by suction, and dried to obtain 100.68g of white crystal compound III-a, the content of 99.30%, yield 80.78%, compound Ⅲ-a, 1HNMR (400MHz, CDCl3) δ8.05 (s, 1H), 7.75 (d, J=8.4Hz, 1H), 4.73 (s, 2H), 2.60 (s , 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com