Bovine parainfluenza virus recombinant antigen and application thereof
A bovine parainfluenza virus, recombinant antigen technology, applied in the direction of virus antigen components, viruses, virus peptides, etc., can solve the problems of poor immune effect, reversion, short immune period, etc., to increase immunogenicity, long immune period, Strong immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the bovine parainfluenza virus recombinant antigen according to one embodiment of the present invention comprises the following steps: cultivating the above-mentioned host cells under suitable conditions, collecting the culture medium and / or the lysate of the host cells, and then separating and purifying to obtain bovine parainfluenza virus Viral Recombinant Antigen.
[0044] In a specific example, the separation and purification method includes one or more of ion exchange chromatography, hydrophobic chromatography, affinity chromatography and molecular sieve, which are not limited thereto and can be selected according to needs.
[0045] The bovine parainfluenza vaccine according to an embodiment of the present invention comprises the above-mentioned bovine parainfluenza virus recombinant antigen and a pharmaceutically acceptable adjuvant.
[0046] In a specific example, the adjuvant can be one or a combination of two or more of MONTANIDE ISA 2...
Embodiment 1
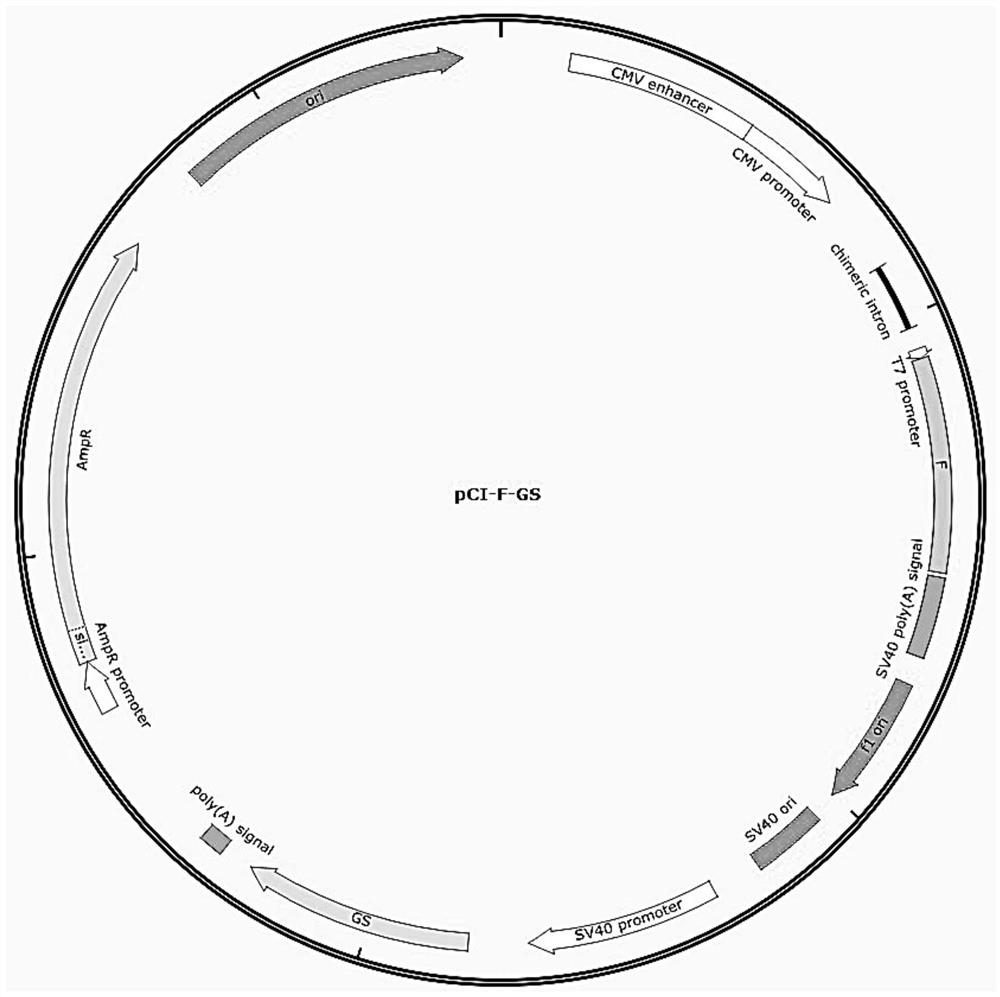
[0049] Embodiment 1 Construction of recombinant eukaryotic expression vector pCI-F-GS
[0050] 1. F gene amplification and purification
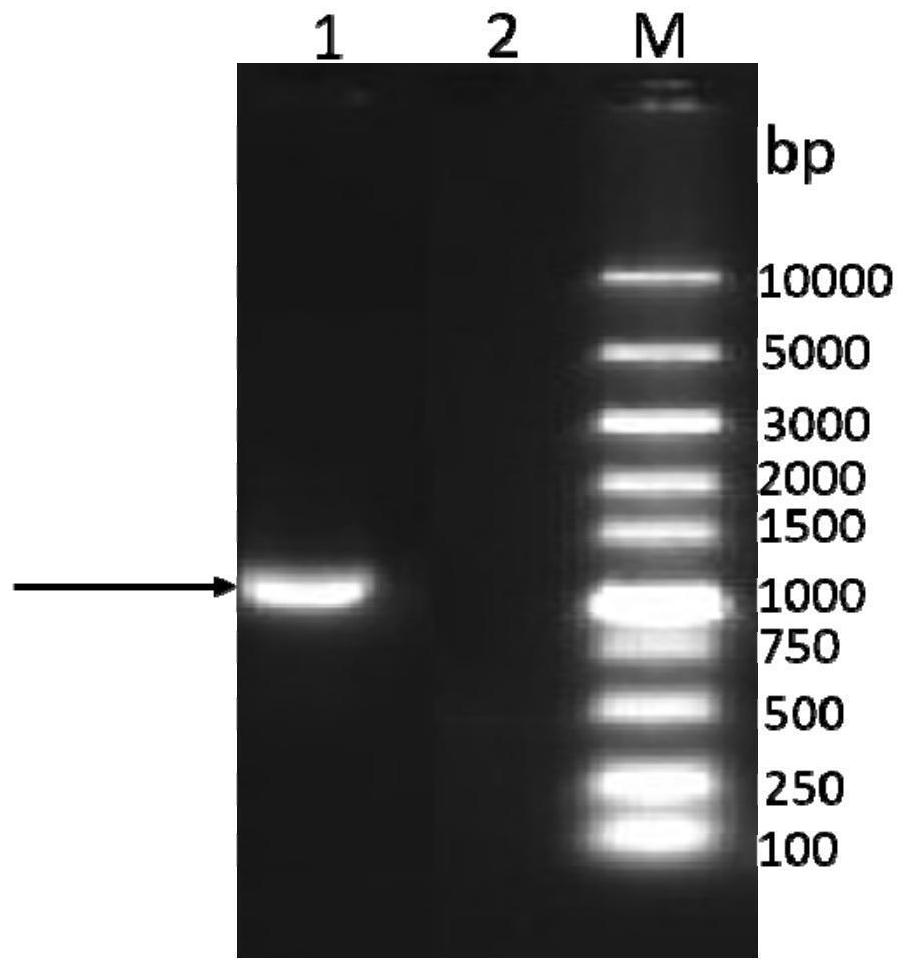
[0051] The codon-optimized F gene (SEQ ID NO: 3) was synthesized in Nanjing GenScript Biotechnology Co., Ltd. and cloned into the pUC-57 vector to obtain the pUC-F plasmid vector. Use pUC-F as a template and use F-F and F-R shown below as primers for PCR amplification. The amplification system is shown in Table 1. The reaction conditions were: pre-denaturation at 94°C for 5 minutes; denaturation at 95°C for 45 seconds, renaturation at 60°C for 45 seconds, extension at 72°C for 2 minutes, 30 cycles; extension at 72°C for 10 minutes, and storage at 4°C.
[0052] F-F: 5'-atactcgagatgttttttggcgaaattatt-3'
[0053] F-R: 5'-ataggtaccttatttgcccgcgctttt-3'
[0054] Table 1 F gene amplification system
[0055] reactive component Unit (μL) pUC-F plasmid 1 F-F primer 1 F-R primer 1 2×PCR MasterMix 12.5 wxya ...
Embodiment 2
[0072] Example 2 Construction of recombinant eukaryotic expression vector pCI-F-HN-GS
[0073] 1. Amplification and purification of HN gene expression cassette
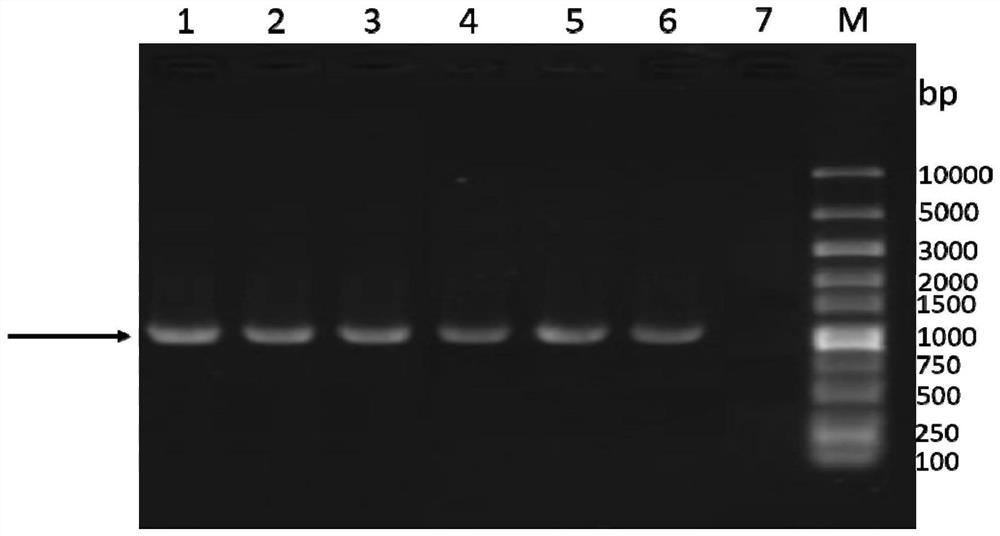
[0074] The codon-optimized HN gene expression cassette (SEQ ID NO: 4) was synthesized in Nanjing GenScript Biotechnology Co., Ltd. and cloned into the pUC-57 vector to obtain the pUC-HN plasmid vector. Using pUC-HN as a template and HN-F and HN-R shown below as primers for PCR amplification, the amplification system is shown in Table 5. The reaction conditions were: pre-denaturation at 94°C for 5 minutes; denaturation at 95°C for 45 seconds, renaturation at 60°C for 45 seconds, extension at 72°C for 2 minutes, 30 cycles; extension at 72°C for 10 minutes, and storage at 4°C.
[0075] HN-F: 5'-ataggtaccatgcagctgtgcagcaccccg-3'
[0076] HN-R: 5'-atactcgagttatttgcccgcgcttttgctggt-3'
[0077] Table 5 HN gene expression cassette amplification system
[0078]
[0079]
[0080] Perform gel electrophoresis on the PCR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com