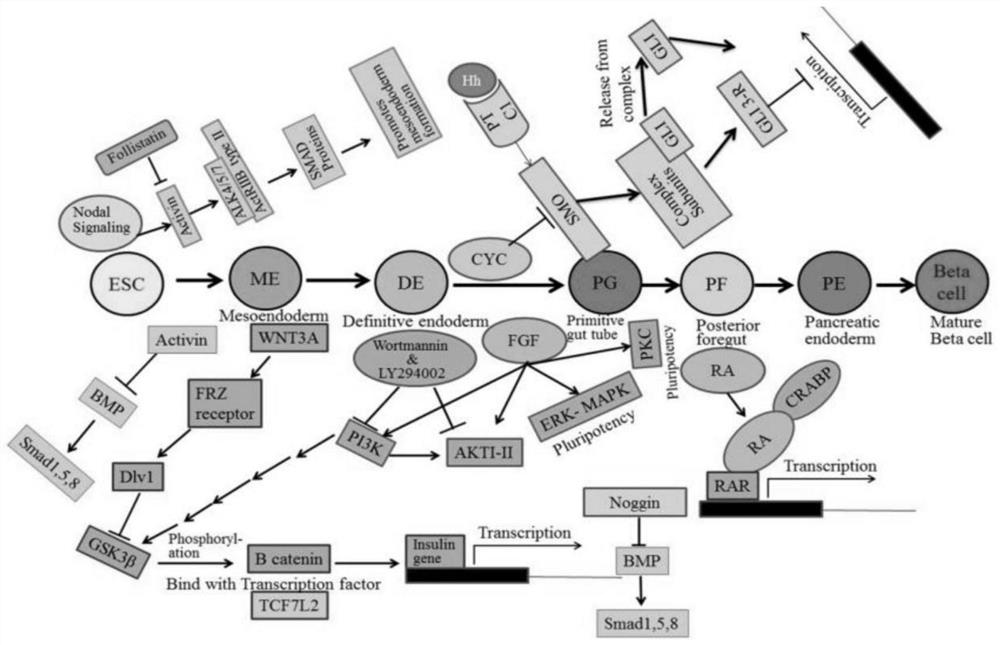
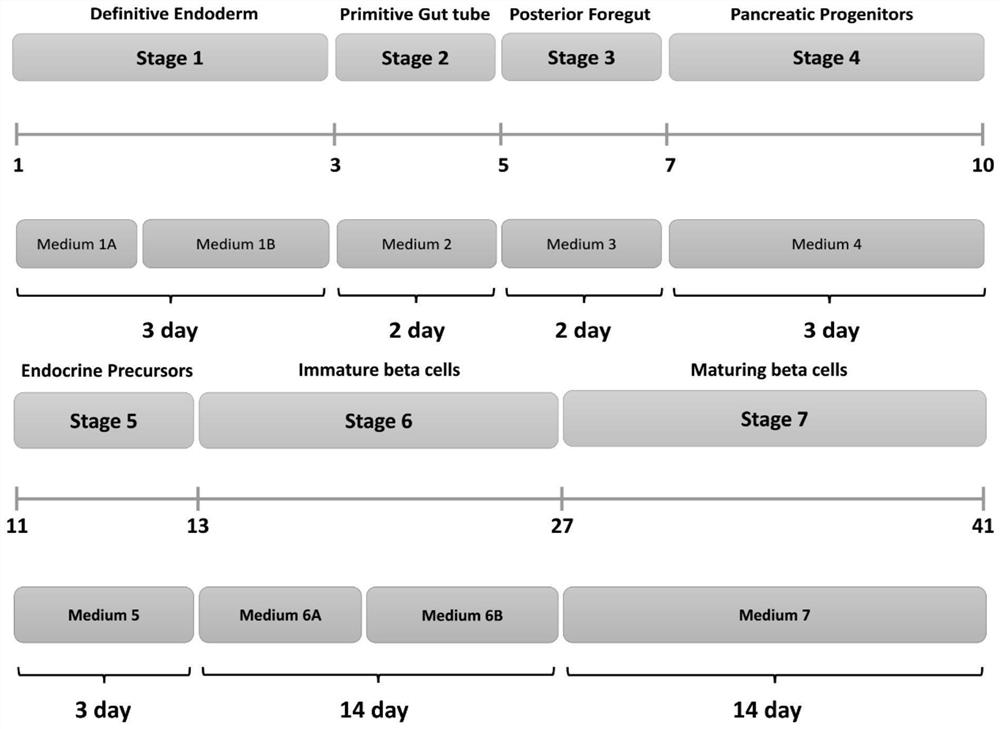
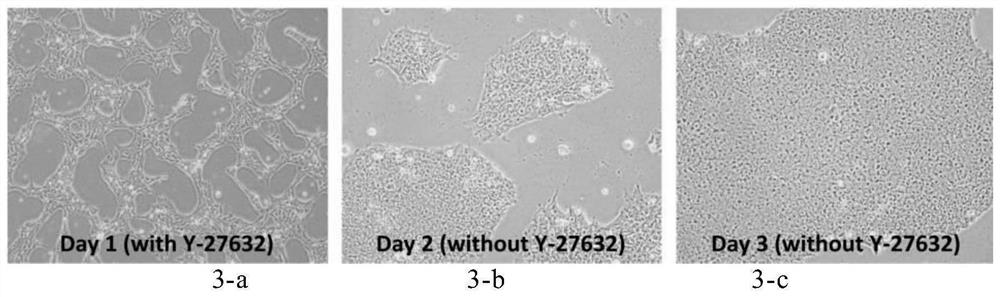
Culture media and applications thereof, and differentiation methods of induced pluripotent stem cells into pancreatic islets
A medium and cell technology, applied in the field of stem cells, can solve the problems affecting the clinical application of induced pancreatic islets, low repeatability, and long differentiation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0471] Stage1: Differentiation of iPSCs to Committed Endoderm
[0472] First, the Matrigel matrix was plated on a cell culture plate, and E8 complete medium was added, and placed in a cell culture incubator for one day to obtain a plated cell culture plate, and then the E8 medium was discarded.
[0473] Use E8 medium to inoculate iPS cells to a cell density of 150,000-200,000 / cm 2 , when the cell confluency reached over 90%, the medium was replaced with stage1 medium A (containing 100ng / ml GDF8, 3μmol / L CHIR-99021, 3g / L sodium bicarbonate, 2mmol / L (100×) glutamine , 20mmol / L glucose, 2% BSA (fetal bovine albumin) in MCDB131 medium), induced 1d.
[0474] On the 2nd day, replace the culture with stage1 medium B (containing 100ng / ml GDF8, 3g / L sodium bicarbonate, 2mmol / L (100×) glutamine, 20mmol / L glucose, 2% BSA (fetal bovine albumin) MCDB131 medium) induced 1d;
[0475] On the 3rd day, replace with fresh stage1 medium B (containing 100ng / ml GDF8, 3g / L sodium bicarbonate, 2mm...
Embodiment 2
[0481] Stage 2: Differentiation of directed endoderm to gastrula tube
[0482] On the 4th day, the culture medium of endoderm cells induced in Example 1 was replaced with stage2 medium: containing 50ng / ml FGF7, 0.25mmol / L ascorbic acid, 3g / L sodium bicarbonate, 2mmol / L (100×) glutamine Amide, 20mmol / L glucose, 2% BSA (fetal bovine albumin) MCDB131 medium, replace fresh culture medium every day, induce 2 days, and obtain original intestinal tube cells.
[0483] After the above 2-day induction, the cells became denser and thicker, and grew in layers. It can be seen that the nuclear-to-cytoplasmic ratio of the cells almost disappeared, and the gaps between the cells showed a raised shape, and cord-like and spherical structures began to appear. ( Image 6 ).
[0484] The induced cells are detected with FOXA2 and HNF1B as markers, and the positive rate of HNF1B of the obtained endoderm cells should not be lower than 80%, and the positive rate of FOXA2 should not be lower than 90%...
Embodiment 3
[0488] Stage 3: Differentiation of protogut tube to posterior foregut
[0489] On the 6th day, the culture medium of endoderm cells induced in Example 2 was replaced with stage3 medium: containing 50ng / ml FGF7, 0.25mmol / L ascorbic acid, 0.25μmol / L SANT-1, 1μmol / L RA, 100nmol / L LDN193189, 2mmol / L ITS-X, 200nmol / L TPB, 3g / L sodium bicarbonate, 2mmol / L (100×) glutamine, 20mmol / L glucose, 2% BSA (fetal bovine albumin) of MCDB131 Medium. Fresh culture medium was replaced every day, induced for 2 days, and hind-foregut cells were obtained.
[0490] After the above 2-day induction, it can be clearly observed that the flat cells at the bottom layer gradually become thinner, and the spherical and ellipsoidal structures gradually increase. Figure 8 .
[0491] In order to control the quality of the obtained cells, the positive rate of PDX1 of the obtained posterior foregut cells should not be lower than 50%, and the positive rate of SOX9 should not be lower than 50%.
[0492] At the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com