A kind of compound lidocaine aerosol and preparation method thereof
A lidocaine and aerosol technology, which is applied in aerosol delivery, anti-inflammatory agents, pharmaceutical formulations, etc., to achieve the effect of quick onset, improvement of blood vessels, and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
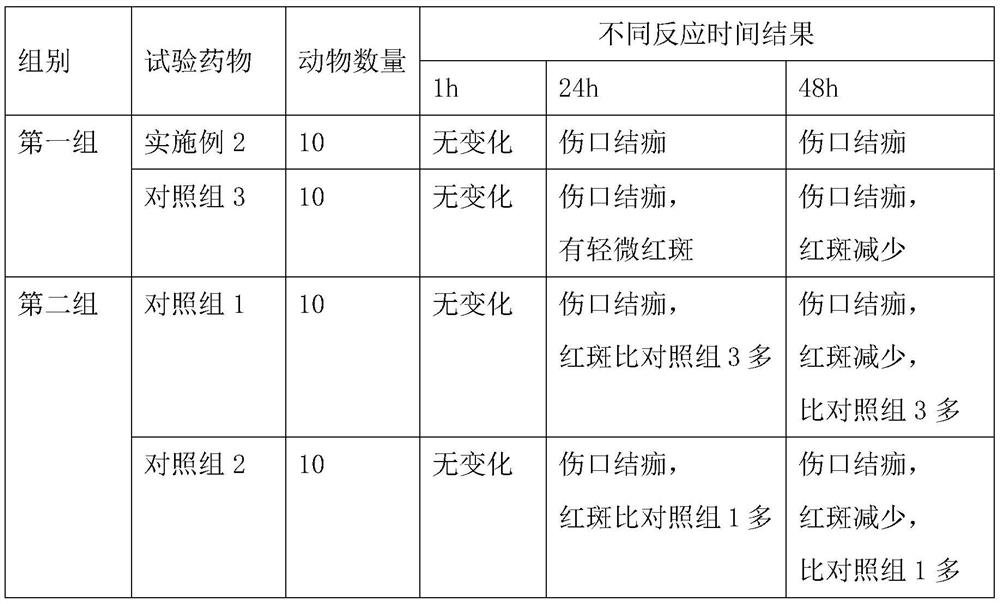
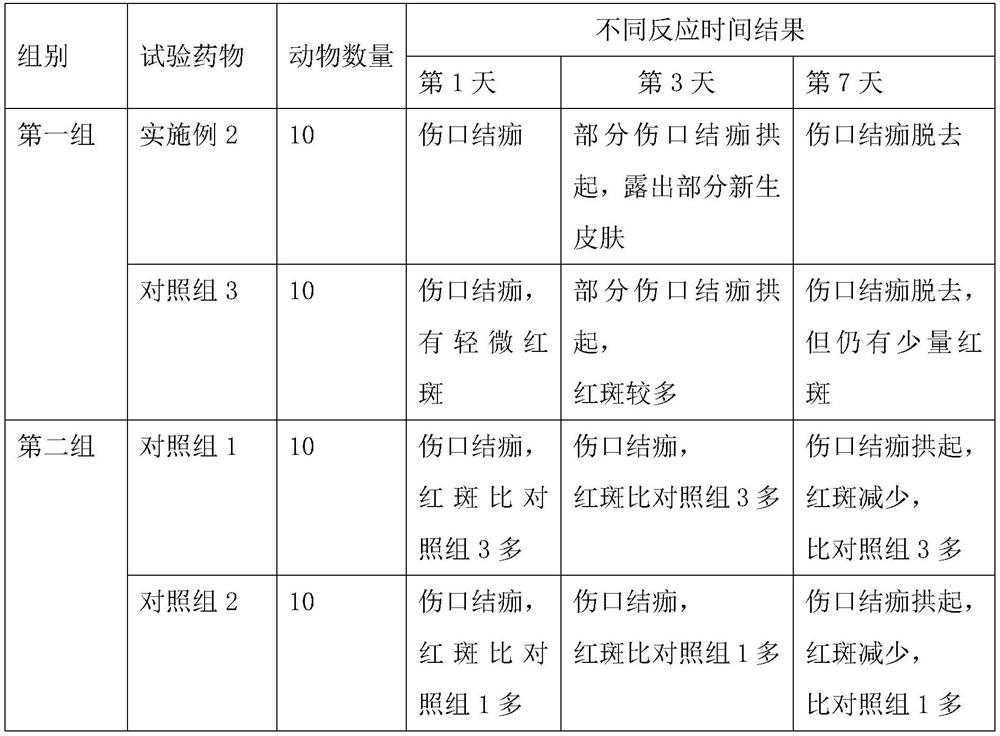
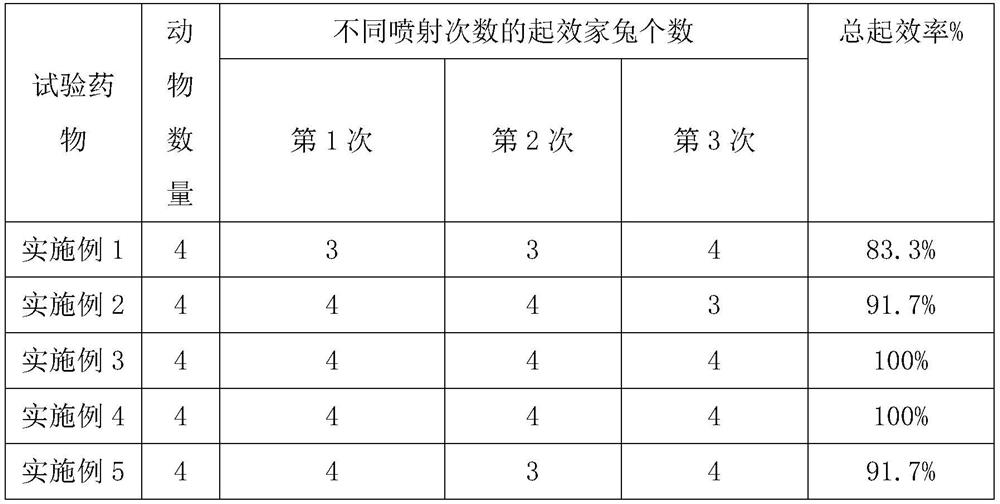
Embodiment 1
[0040] This embodiment relates to a compound lidocaine aerosol, which consists of the following components in parts by weight: 0.2 parts of lidocaine, 0.2 parts of prilocaine, 1 part of 0.1M sodium hydroxide, 2 parts of propylene butane 1 part, 1 part of polyvinyl alcohol with a degree of alcoholysis of 68%, and 0.1 part of sodium rutin.
[0041] A preparation method of compound lidocaine aerosol, the specific steps are as follows.
[0042] a. Add deionized water to polyvinyl alcohol, heat to 78°C, stir until completely dissolved, and cool to room temperature for later use.
[0043] b, the preparation of rutin derivatives:
[0044] b1 Add 9 times the amount of 68% ethanol aqueous solution of Sophora japonica rice to Sophora japonica rice, extract in a water bath with an ultrasonic frequency of 10KHZ for 30 minutes, and separate solid-liquid to obtain the first crude extract;
[0045] b2, adding the filter residue in b1 to 68% ethanol aqueous solution, after ultrasonic leachi...
Embodiment 2
[0050] This embodiment relates to a compound lidocaine aerosol, which consists of the following components in parts by weight: 3.5 parts of lidocaine, 3.5 parts of prilocaine, 3.5 parts of 0.1M sodium hydroxide, 1,1, 15 parts of 1,2-tetrafluoroethane, 11 parts of propane, 17.6 parts of polyvinyl alcohol with an alcoholysis degree of 75%, and 1.75 parts of sodium rutin.
[0051] A preparation method of compound lidocaine aerosol, the specific steps are as follows.
[0052] a. Add deionized water to polyvinyl alcohol, heat to 85°C, stir until completely dissolved, and cool to room temperature for later use.
[0053] b, the preparation of rutin derivatives:
[0054] b1 Add 9 times the amount of 72% ethanol aqueous solution of Sophora japonica rice to Sophora japonica rice, extract in a water bath with an ultrasonic frequency of 30KHZ for 40 minutes, and separate solid-liquid to obtain the first crude extract;
[0055] b2, adding the filter residue in b1 to 68% ethanol aqueous s...
Embodiment 3
[0060] This embodiment relates to a compound lidocaine aerosol, which consists of the following components in parts by weight: 2 parts of lidocaine, 2 parts of prilocaine, 2 parts of 0.1M sodium hydroxide, 1,1, 10 parts of 1,2,3,3,3-heptafluoropropane, 8 parts of propane, 9.5 parts of polyvinyl alcohol with an alcoholysis degree of 75%, and 1 part of sodium rutin.
[0061] A preparation method of compound lidocaine aerosol, the specific steps are as follows.
[0062] a. Add deionized water to polyvinyl alcohol, heat to 85°C, stir until completely dissolved, and cool to room temperature for later use.
[0063] b, the preparation of rutin derivatives:
[0064] b1 Adding 9.5 times the amount of 72% ethanol aqueous solution of Sophora japonica rice to Sophora japonica rice, leaching in a water bath with an ultrasonic frequency of 25KHZ for 36 minutes, and then separating the solid and liquid to obtain the first crude extract;
[0065] b2, adding the filter residue in b1 to 70% e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| alcoholysis degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com