Medical application of recombinant protein Semaphorin3G in prevention and treatment of retinal diseases
A recombinant protein, retinopathy technology, applied in medical preparations containing active ingredients, sensory diseases, pharmaceutical formulations, etc., can solve problems such as the molecular mechanism of vascular development in cells has not yet been elucidated, and achieve improved vascular leakage, reduced area, Fading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
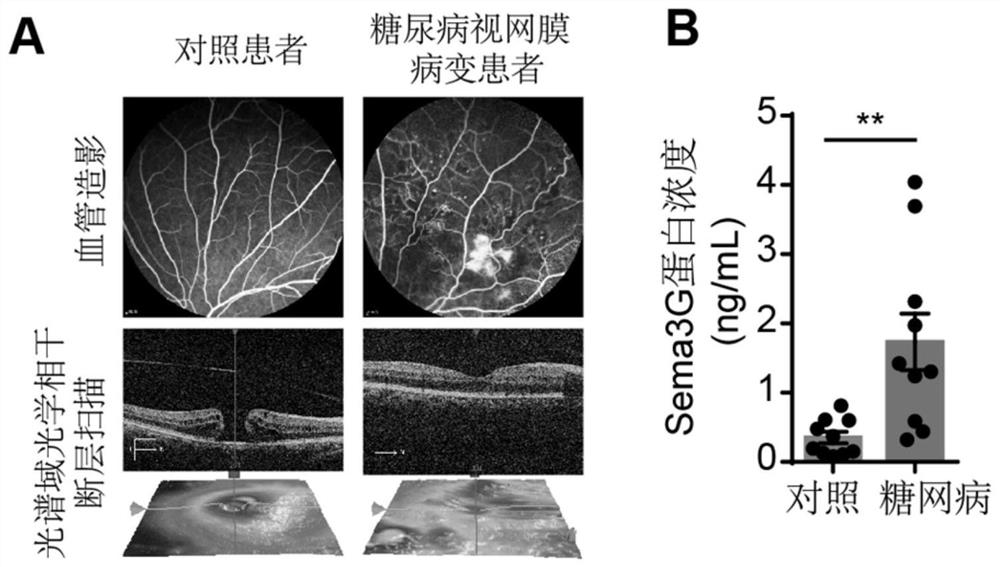
[0036] Example 1 Detection of Sema3G protein content in vitreous cavity fluid of patients with proliferative diabetic retinopathy
[0037] The patients with diabetic retinopathy involved in this experiment were clinically diagnosed as proliferative diabetic retinopathy (PDR) from stage IV to stage VI by the ophthalmologists of Jiangsu Provincial People's Hospital according to the classification criteria of diabetic retinopathy formulated by the Fundus Disease Group of the Chinese Medical Association. Pathological neovascularization in the retina. Control patients had non-vascular pathological retinopathy diseases such as idiopathic macular hole (MH) or idiopathic epiretinal membrane (ERM). Vitreous cavity fluid samples from the above PDR patients and control patients were collected during vitrectomy. All subjects in this study signed a written informed consent, and the research project has been approved by the Medical Research Ethics Committee of Jiangsu Provincial People's H...
Embodiment 2
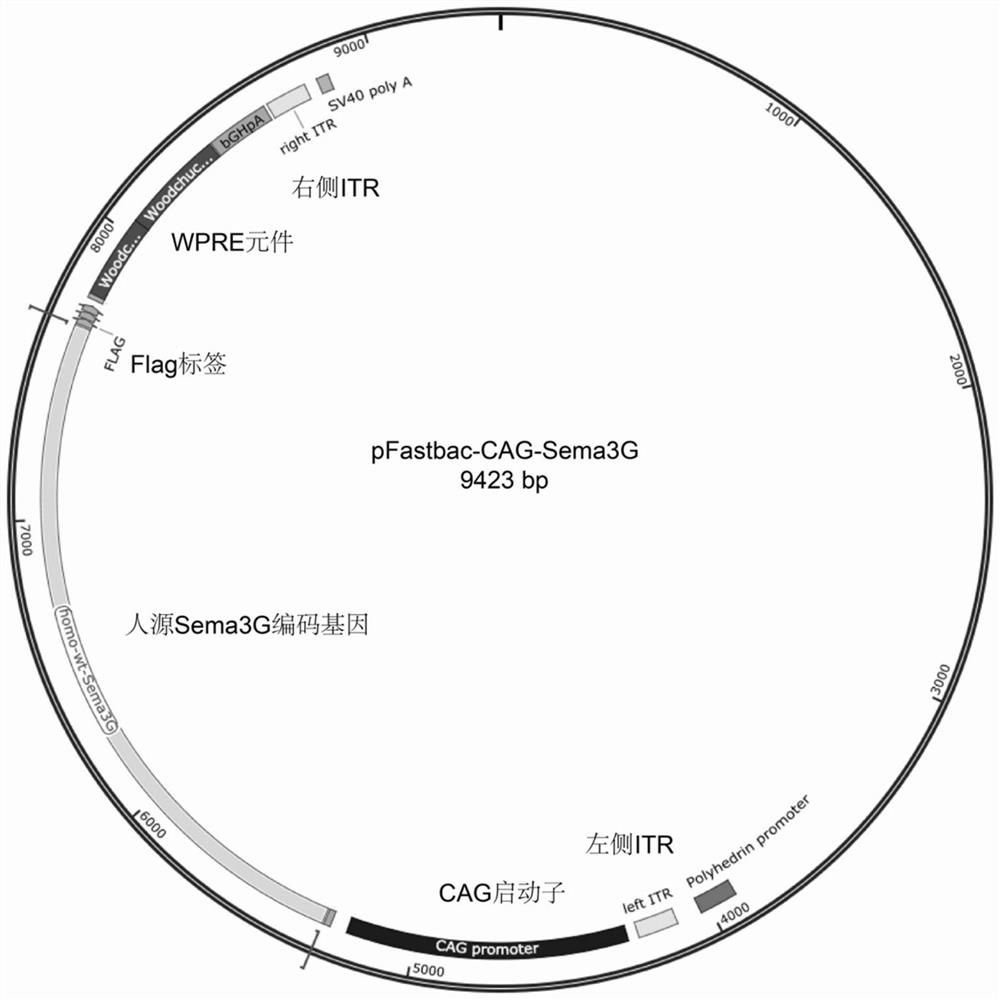
[0054] Example 2 Construction of an adeno-associated virus vector expressing Sema3G protein
[0055] (1) Gene sequence acquisition: Sema3G (NCBI, NM_020163.3) gene sequence and plasmid vector were obtained from the database website, and the Sema3G coding gene was constructed into the BR1 serotype adeno-associated virus backbone vector by using molecular cloning technology.
[0056] (2) PCR amplification and digestion: use the gene sequence-specific primers designed in Table 1.1, and use the Sema3G-encoding gene vector as a template to perform PCR amplification on the target gene sequence to obtain Sema3G gene fragments. Amplification (Gflex PCR enzyme, takara company) program: 95°C, 10min; 95°C, 10s; 60°C, 30s; 68°C, 2min; a total of 35 cycles; 72°C, 4min. The backbone vector was digested with restriction endonucleases EcoR I and Hind III (Takara Company), and reacted in a water bath at 37° C. for 2 hours for digestion.
[0057] Table 1.1
[0058] Primer name Pri...
Embodiment 3
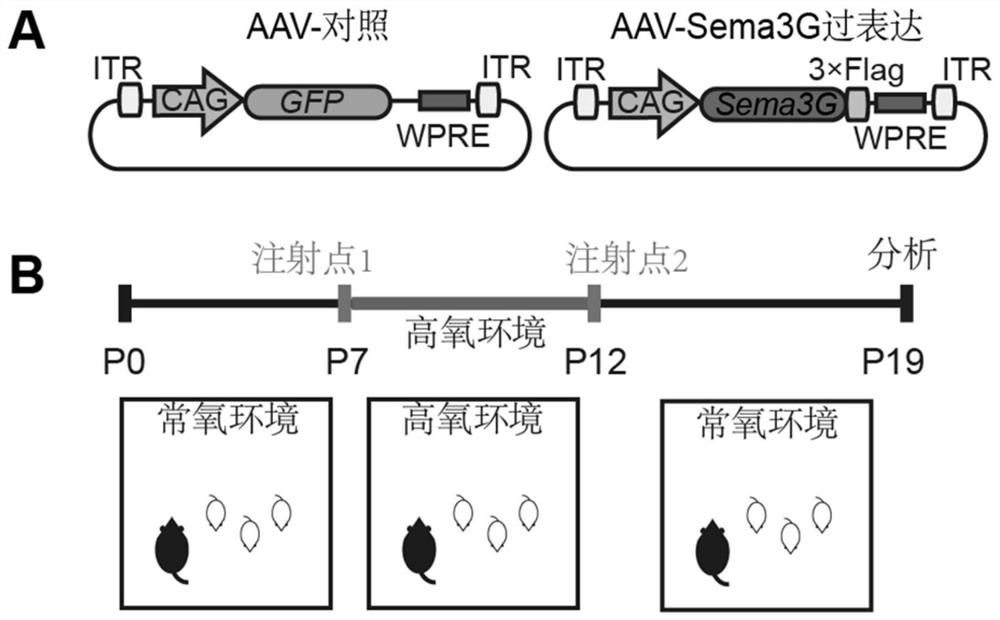
[0064] Example 3 Packaging of adeno-associated virus expressing Sema3G protein
[0065] During the packaging process of AAV, the packaging plasmid is responsible for encoding the target gene and two inverted terminal repeats (ITR), and the helper plasmid pxx2 contains the cap (encodes the viral capsid protein) and rep (involves in the replication of the virus) genes required for AAV packaging. p179 is a helper plasmid packaged by adeno-associated virus. The gene encoding the capsid protein of the helper plasmid we used has NRGTEWD mutation, which can specifically infect the vascular endothelial cells of the central nervous system. After the three plasmids were co-transfected into 293T cells, the AAV virus began to replicate and package. The obtained virus particles were purified by ultracentrifugation, and the virus gene copy was determined by qPCR. Follow-up related experiments were carried out according to the titer of the virus. Virus packaging was completed by Heyuan Bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com