Synthesis method of chiral alpha-amino acid ester derivative
A technology of amino acid esters and synthetic methods, applied in the direction of asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., to achieve the effects of mild conditions, simple operation, and good industrial practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
[0066] Under nitrogen protection, [Ir(COD)Cl] 2 (0.002mmol, 0.5mol%), chiral ferrocene skeleton phosphine-phosphoramidite ligand (0.0048mmol, 1.1mol%) was dissolved in tetrahydrofuran (1.0mL), stirred at room temperature for 10 minutes, added substrate (4- Methoxyphenylimino) tetrahydrofuran (1.0mL) solution of methyl phenylacetate (0.4mmol) and 5.1mg iodine are placed in an autoclave, replaced by hydrogen for 3 times, and then fed with hydrogen to 50 atmospheres , reacted at 25°C for 24 hours. Hydrogen gas was released slowly, and the product was separated with a silica gel column after removing the solvent.
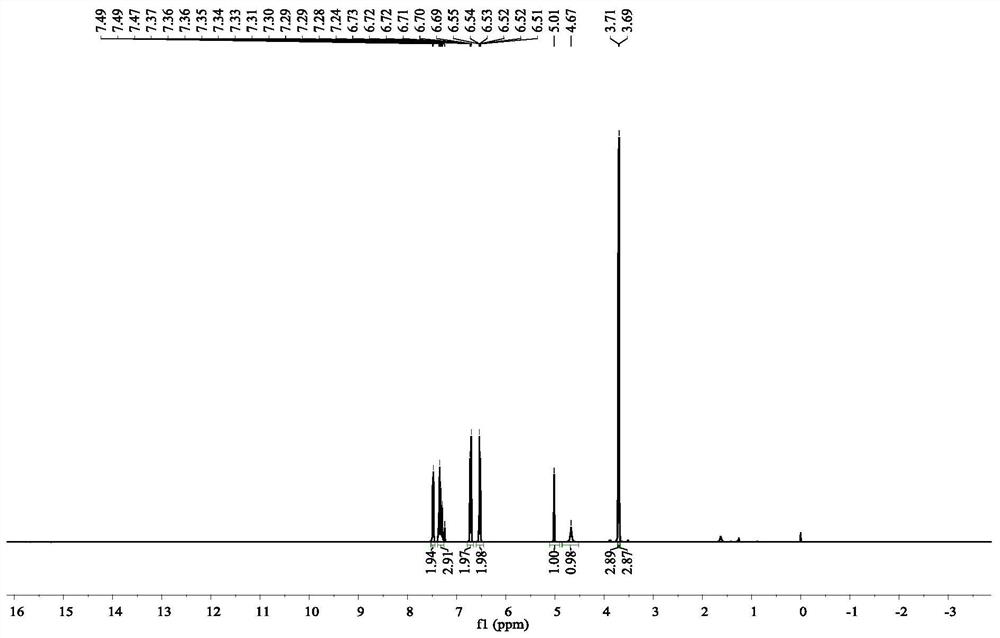
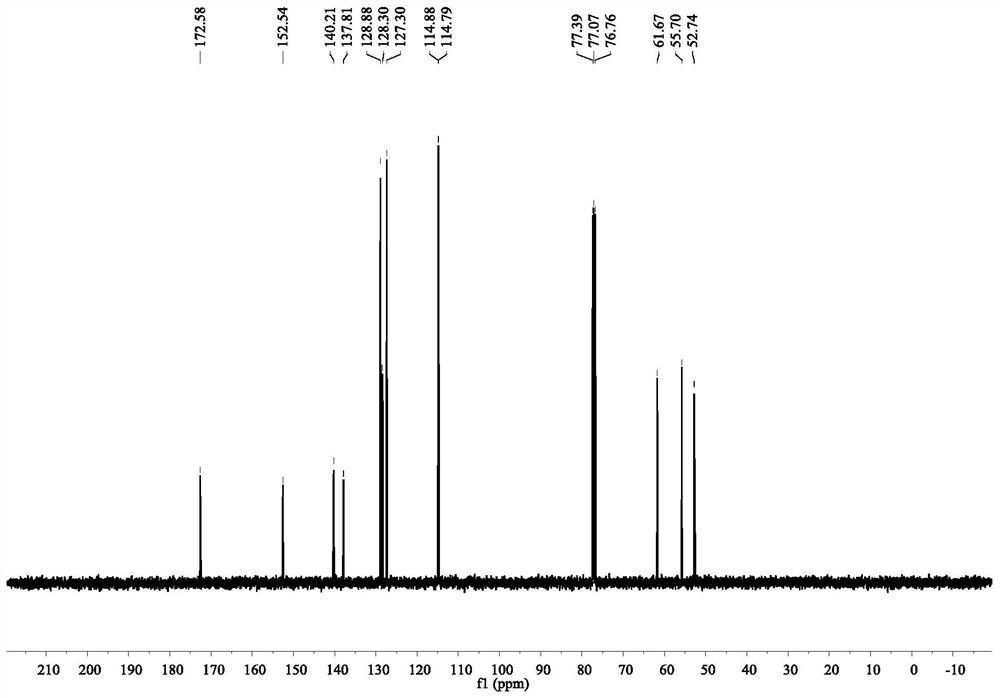
[0067] The product is detected and analyzed, and the NMR and HPLC data are as follows:
[0068] Yellow oil was obtained in 96% yield after purification with column chromatography on silica gel (hexanes / ethyl acetate,10 / 1).92%ee was determined by chiral HPLC (Chiralcel OJ-H, n-hexane / i-PrOH=70 / 30 ,0.8ml / min,254nm,40℃):t R (minor) = 26.8min,t R (major) = ...
Embodiment 2
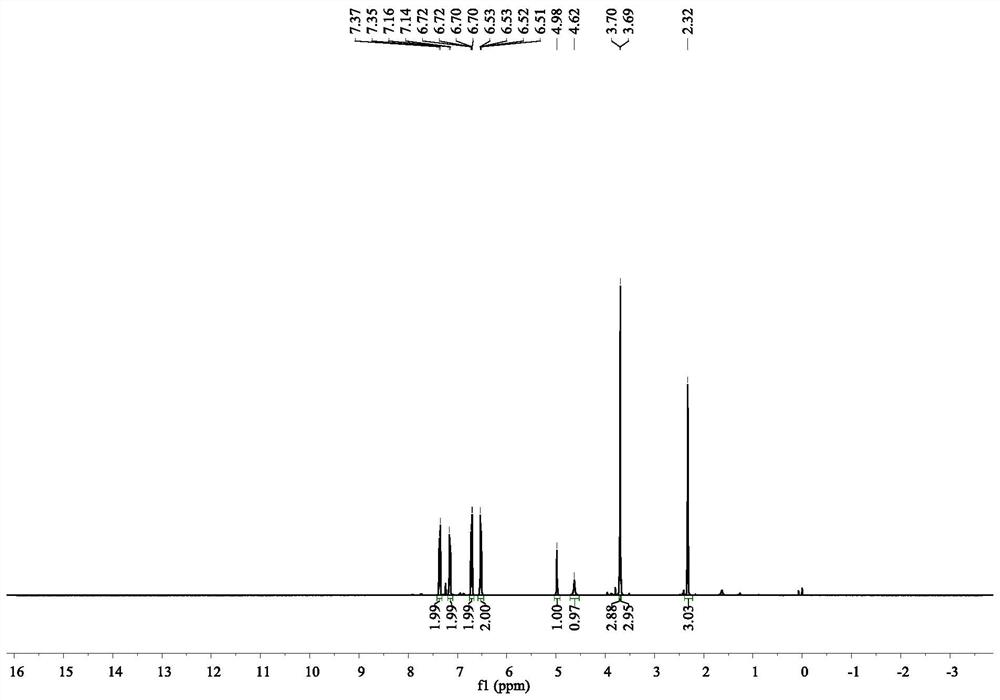
[0071] With the reaction condition H among the embodiment 1 2 The pressure was changed to 100 atmospheres, and the rest were the same as in Example 1 to obtain the product. After testing, the product was (4-methoxyanilino) methyl phenylacetate, with a yield of 98% and an enantioselectivity of 92% ee.
Embodiment 3
[0073] With the reaction condition H among the embodiment 1 2 The pressure was changed to 20 atmospheres, and the rest were the same as in Example 1 to obtain the product. After testing, the product was (4-methoxyanilino)methyl phenylacetate, with a yield of 76% and an enantioselectivity of 90% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com