Synthetic method of ethyl 4, 4-difluoro-3-oxo-2-piperidine-1-yl methylene butyrate
A technology of ethyl methylene butyrate and a synthesis method is applied in the field of compound synthesis and can solve the problems of low utilization rate of fluorine atoms, high requirements for labor protection safety facilities, and difficulty in controlling volatile organic compounds and nitrogen oxides. problems, to achieve the effect of protecting the ecological environment, realizing recyclable use, and facilitating labor protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of complex catalyst among the present invention can refer to following documents and books:
[0042] (1) Efficient, Single-Step Access to Imidazo [1,5-a] pyridine N-Heterocyclic Carbene Precursors [J]. ORGANIC LETTERS. 2011 Vol.13, No.19 5256–5259;
[0043] (2) (C ∧ C * )-cyclometalated platinum(II) imidazo [1,5-a] pyridine NHCcomplexes-Synthesis and characterization[J]. Journal of Organometallic Chemistry. 775(2015). 155-163;
[0044] (3) Efficient synthesis of bulky N-Heterocyclic carbene ligands forcoinage metal complexes[J]. Journal of Organometallic Chemistry. 820(2016).1-7;
[0045] (4) Synthesis and characterization of novel cyclopentadienyl molybdenumimidazo [1,5-a] pyridine-3-ylidene complexes and their application in olefinepoxidation catalysis[J]. Journal of Catalysis. 319(2014). 119–126;
[0046] (5) Chiral imidazo [1,5-a] tetrahydroquinoline N-heterocyclic carbenes and their copper complexes for asymmetric catalysis[J]. Tetrahedron...
Embodiment 1
[0050] The specific steps of the synthetic method of the 4,4-difluoro-3-oxo-2-piperidin-1-yl methylene butyrate ethyl ester of the present embodiment are:
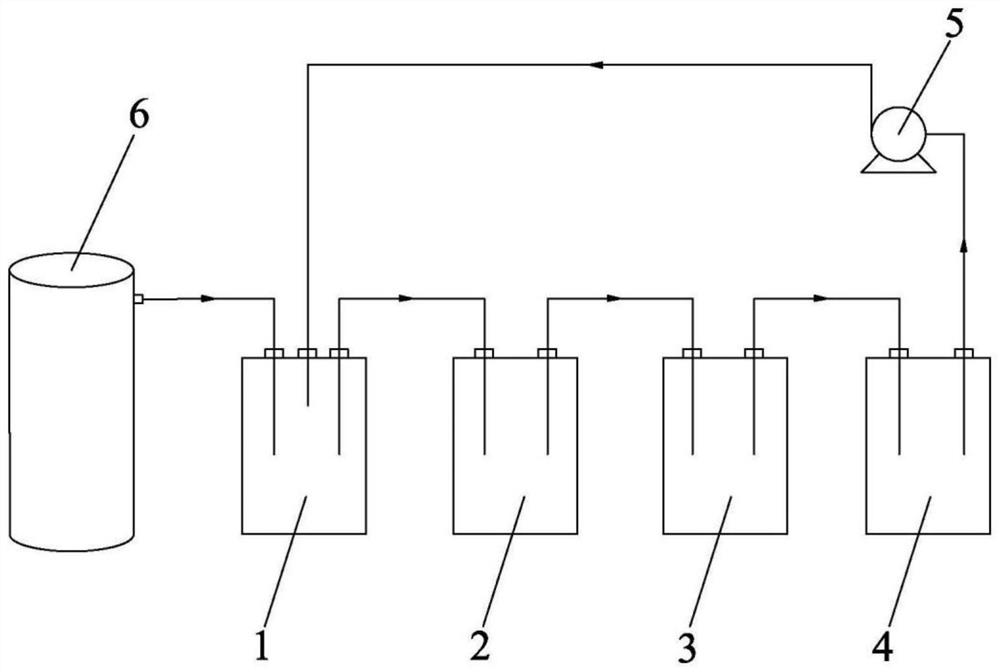
[0051] use as figure 1 The production device shown, first 3-(1-piperidinyl)-ethyl acrylate (800 kg, 4.37 kmoL), 1-fluoro-2-fluoromethylpiperidine (30 kg, 0.22 kmoL) and trichloroethylene (1988.35 kg, 15.3 kmoL) was sequentially added to the 5000L Hastelloy condensation reactor 1, the motor was turned on to stir, and the temperature was lowered to below 5°C for later use, that is, the liquid to be condensed.
[0052] Then continuously pump tetrafluoroethyl ether gas into the cracker 6, and the cracked gas (mainly composed of difluoroacetyl fluoride, ethylene, and hydrogen fluoride) generated by the cracking is passed into the 5000 L Hastelloy condensation reactor 1 to the condensation reactor 1 The 3-(1-piperidinyl)-ethyl acrylate in the reaction is completed and the pumping is stopped. The reaction formula that reacts in...
Embodiment 2
[0073] The specific steps of the synthetic method of the 4,4-difluoro-3-oxo-2-piperidin-1-yl methylene butyrate ethyl ester of the present embodiment are:
[0074] First, 3-(1-piperidinyl)-ethyl acrylate (1000 kg, 5.46 kmoL), 1-fluoro-2-fluoromethylpiperidine (33.33 kg, 0.24 kmoL) and trichloroethylene (2500 kg, 19.24 kmoL) into the 5000 L Hastelloy Condensation Reactor 1 in turn, turn on the motor to stir, stir and cool down to below 4°C for later use, that is, the liquid to be condensed.
[0075] Then continuously pump tetrafluoroethyl ether gas into the cracker 6, and the cracked gas (mainly composed of difluoroacetyl fluoride, ethylene, and hydrogen fluoride) generated by the cracking is passed into the 5000 L Hastelloy condensation reactor 1 to the condensation reactor 1 The 3-(1-piperidinyl)-ethyl acrylate in the reaction is completed and the pumping is stopped.
[0076] Difluoroacetyl fluoride and 3-(1-piperidinyl)-acrylic acid ethyl ester carry out condensation reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com