Synthetic method of organic DSTMS crystal high-purity growth raw material
A synthesis method and organic technology, applied in the field of synthesis of high-purity organic DSTMS crystal growth raw materials, can solve the problems of reduced process cost, complex reaction process, and low product purity, and achieve the effects of low cost, simple preparation method, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
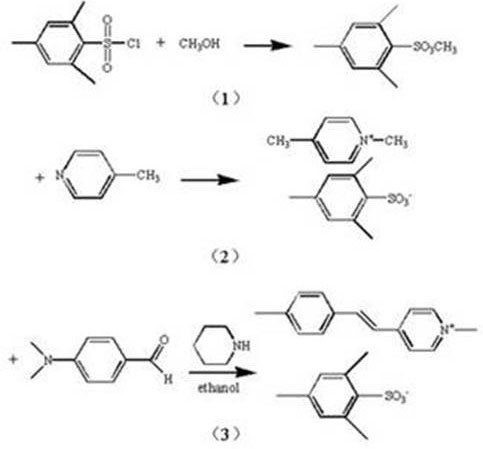
[0033] 21.87g (0.1mol) of mesitylenesulfonyl chloride was dissolved in 301mL of methanol, 1.4mL (14.13umol) of organic base piperidine was added dropwise, mixed with it, stirred, reacted at room temperature for 4 hours, and the excess solvent was removed by rotary evaporation to obtain 2 , 4,6-trimethylbenzenesulfonate; then 9.89ml (0.1mol, 9.31g) of 4-picoline and 18.65mL of 2,4,6-trimethylbenzenesulfonate (0.1mol, 21.43 g) Dissolve in 50ml of absolute ethanol successively, pour into a three-necked flask, put magnets in a water bath constant temperature magnetic stirrer, heat and stir, set the temperature at 48 ℃, condense and reflux for 8 hours, and generate an intermediate product; Finally, dissolve 14.92g (0.1mol) of p-dimethylaminobenzaldehyde in 120ml of absolute ethanol, then mix it with the intermediate product from the previous step, drop 500uL of organic base piperidine as a catalyst, and set the temperature at 75°C , reacted for 12 hours, and ended the reaction when...
Embodiment 2
[0035]Dissolve 30g (0.136mol) of mesitylenesulfonyl chloride in 410mL of methanol, add dropwise 1.8mL (18.17umol) of organic base piperidine, mix with it, stir, react at room temperature for 6 hours, remove excess solvent by rotary evaporation to obtain 2, 4,6-trimethylbenzenesulfonate; then 13.45ml (0.136mol, 12.66g) of 4-picoline and 25.36mL (0.136mol, 29.14g) of 2,4,6-trimethylbenzenesulfonate ) were successively dissolved in 68ml of absolute ethanol, poured into a three-necked flask, placed in a magnet and placed in a constant temperature magnetic stirrer in a water bath, heated and stirred, the temperature was set at 50°C, condensed and refluxed for 10 hours, and an intermediate product was generated; finally Dissolve 20.29g (0.136mol) of p-dimethylaminobenzaldehyde in 165ml of absolute ethanol, then mix it with the intermediate product from the previous step, drop 700uL of organic base piperidine as a catalyst, and set the temperature at 78°C. After 16 hours of reaction,...
Embodiment 3
[0037] 15g (0.068mol) of mesitylenesulfonyl chloride was dissolved in 205mL of methanol, 800uL (8.08umol) of organic base piperidine was added dropwise, mixed with it, stirred, reacted at room temperature for 3 hours, and the excess solvent was removed by rotary evaporation to obtain 2,4 , 6-trimethylbenzenesulfonate; then 6.73ml (0.068mol, 6.33g) of 4-picoline and 12.68mL (0.068mol, 14.57g) of 2,4,6-trimethylbenzenesulfonate Dissolved in 50ml of absolute ethanol successively, poured into a three-necked flask, placed in a magnet and placed in a constant temperature magnetic stirrer in a water bath, heated and stirred, the temperature was set at 45°C, condensed and refluxed for 8 hours, and an intermediate product was generated; finally Dissolve 14.92g (0.068mol) of p-dimethylaminobenzaldehyde in 100ml of absolute ethanol, then mix it with the intermediate product from the previous step, drop 500uL of organic base piperidine as a catalyst, set the temperature at 70°C, and react ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com