Method for epoxidizing macrocyclic olefin by hydrogen peroxide method
A technology of macrocyclene and hydrogen peroxide, which is applied in the direction of organic chemistry, can solve the problems of difficulty in recycling catalyst raw materials, difficulty in balancing conversion rate and selectivity, and strong limitation of hydrogen peroxide concentration, so as to overcome the limitation of hydrogen peroxide concentration and make the reaction process green Environmental protection, wide concentration range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
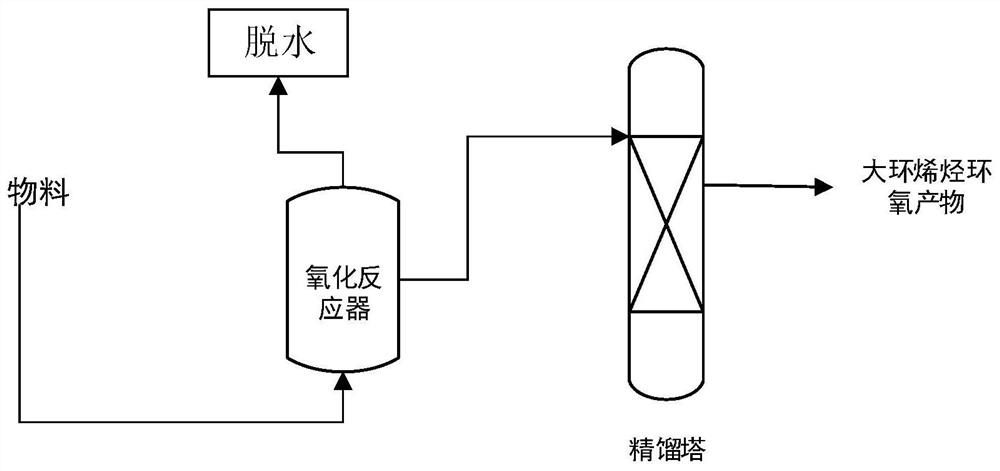
[0036] 68.0Kg of 50% hydrogen peroxide, 3.4Kg of trisodium phosphate and 1.1Kg of ethylenediaminetetraacetic acid are mixed evenly and put into the head tank A, 1102.0Kg of cyclooctene and 110.2Kg of tert-butanol are put into the head tank B, and the head tank A and The materials in B are added to the 2 cubic reaction kettle within 0.5 hours, the steam pressure is adjusted during the feeding process, and the temperature of the kettle is maintained at 75°C. The volatilization pipe of the reaction kettle is connected to a 1-meter BX packed rectification tower, and the vacuum degree is controlled at 1000Pa. The tower top effluent has a hydrogen peroxide content of 3.0%, and the water content of the material in the reactor is 1.5%. The hydrogen peroxide content of the material in the reactor is 0.5%, the utilization rate of hydrogen peroxide is 83.2%, and the molar yield of epoxy cyclooctane corresponding to cyclooctene is 89.5%.
Embodiment 2
[0038] Mix 1000Kg of 5% hydrogen peroxide, 1Kg of sodium tripolyphosphate and 22.1Kg of diethylamine pentaacetic acid evenly into the high-level tank A, and put 442.1Kg of cyclotetracene and 221.1Kg of tert-amyl alcohol into the high-level tank B, and the high-level tank A Add the materials in B and B to a 3-cubic reaction kettle within 6 hours, adjust the steam pressure during the feeding process, and keep the kettle temperature at 120°C. The volatilization pipe of the reaction kettle is connected to a 1-meter BX packed rectification tower, and the vacuum degree is controlled to 7000Pa. The tower top effluent has a hydrogen peroxide content of 0.01%, and the water content of the materials in the reactor is 0.1%. The hydrogen peroxide content of the material in the reactor is 0.01%, the utilization rate of hydrogen peroxide is 97.1%, and the molar yield of epoxy cyclotetradecane corresponding to cyclotetracocene is 96.5%.
Embodiment 3
[0040] 100Kg of 80% hydrogen peroxide, 1.5Kg of sodium polyphosphate and 12.1Kg of N-hydroxyethyldiaminetriacetic acid are mixed evenly into the head tank A, 560.2Kg of cyclohexadecene and 221.1Kg of tert-butanol are put into the head tank B , the materials in the head tanks A and B are added to a 1 cubic reaction kettle within 1.5 hours, the steam pressure is adjusted during the feeding process, and the temperature of the kettle is maintained at 90°C. The volatilization pipe of the reaction kettle is connected to a 1-meter BX packed rectification tower, and the vacuum degree is controlled at 3000Pa. The tower top effluent has a hydrogen peroxide content of 0.12%, and the water content of the materials in the reactor is 0.13%. The hydrogen peroxide content of the material in the reactor is 0.11%, the utilization rate of hydrogen peroxide is 97.4%, and the molar yield of epoxycyclohexadecane corresponding to cyclohexadecene is 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com