Ceftolozane sulfate and preparation method and application thereof
A technology of ceftolozax and sulfuric acid, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as harsh reaction conditions, low safety, and environmental pollution, and achieve the effects of controllable reaction, less pollutant generation, and simplified and optimized reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 provides a kind of preparation method of ceftolozax sulfate, comprises the steps:
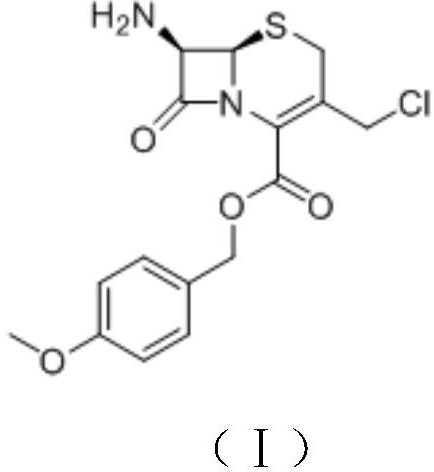
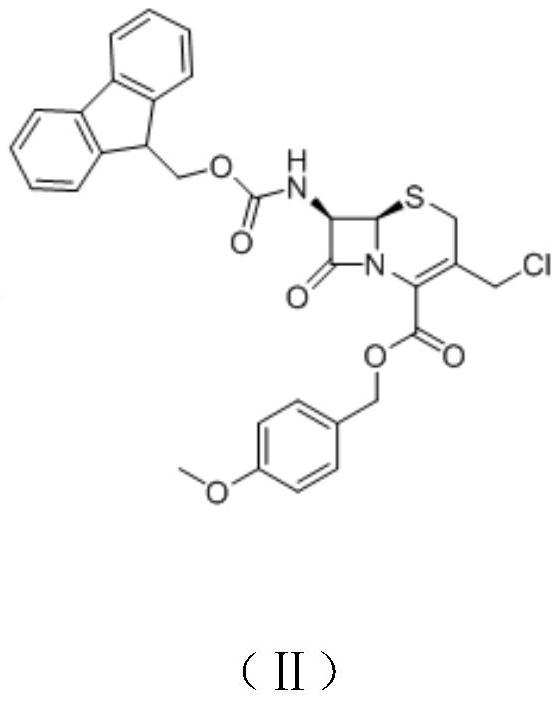
[0061] A) Preparation of Intermediate II
[0062] Intermediate I (20.0g, 54mmol) was dissolved in dichloromethane (250mL), 10% sodium bicarbonate (70g, 83mmol) solution was added, cooled in an ice bath, fluorenylmethoxycarbonyl chloride (16.0g, 62mmol) was added slowly, 0 Reaction at ℃ for 6 h, rotary evaporation to dryness under reduced pressure, extraction with dichloromethane, washing with brine, drying over anhydrous sodium sulfate, rotary evaporation to dryness under reduced pressure, the obtained crude product was recrystallized from a mixed solvent of ethyl acetate-petroleum ether, Intermediate II was obtained as an off-white to solid (31.5 g), with a yield of 98%.
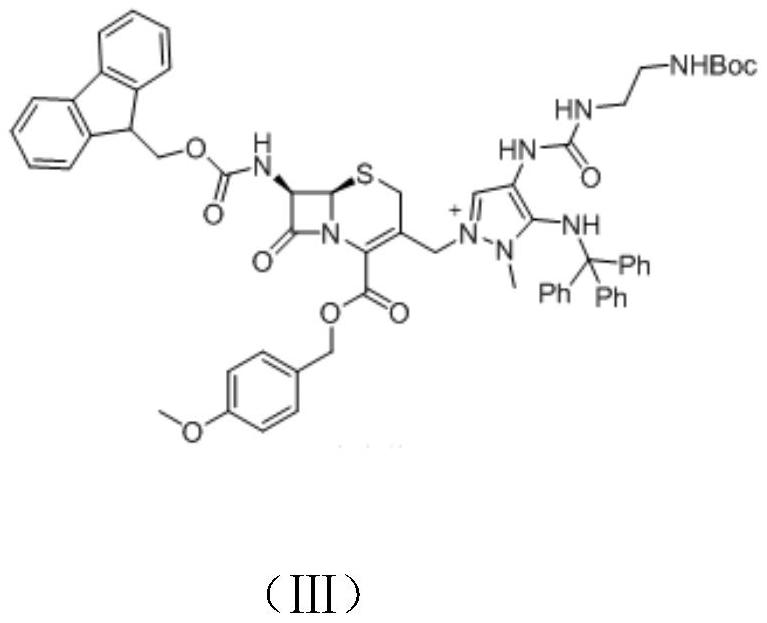
[0063] B) Preparation of Intermediate III
[0064] {2-[3-(1-Methyl-5-(tritylamino)-1H-pyrazol-4-yl)ureido]ethyl}carbamate tert-butyl ester (30.0g, 55mmol) was dissolved in N,N-Dimethylformamide (500mL)...
Embodiment 2
[0070] Embodiment 2 provides a kind of preparation method of ceftolozax sulfate, comprises the steps:
[0071] A) Preparation of Intermediate II
[0072] Intermediate I (25.0g, 68mmol) was dissolved in 1,2-dichloroethane (300mL), added 10% sodium carbonate (122g, 115mmol) solution, cooled in an ice bath, and slowly added fluorenylmethoxycarbonyl chloride (23.0g, 89mmol), reacted at 5°C for 3h, evaporated to dryness under reduced pressure, extracted with dichloromethane, washed with brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. Crystallization gave Intermediate II as an off-white to solid (39.0 g), yield 97%.
[0073] B) Preparation of Intermediate III
[0074] {2-[3-(1-Methyl-5-(tritylamino)-1H-pyrazol-4-yl)ureido]ethyl}carbamate tert-butyl ester (43.0g, 80mmol) was dissolved in N, N-dimethylformamide (600mL), add hexamethyldisilazide (47.0g, 0.23mol), then add intermediate II (39.0g, 66mmol) and potassium iodide (20.0g, 0.12m...
Embodiment 3
[0080] Embodiment 3 provides a kind of preparation method of ceftriaxone sulfate, comprises the steps:
[0081] A) Preparation of Intermediate II
[0082] Intermediate I (85.0g, 0.23mol) was dissolved in chloroform (1000mL), 10% potassium carbonate (630.0g, 0.46mol) solution was added, cooled in an ice bath, fluorenylmethoxycarbonyl chloride (88.0g, 0.34mol) was added slowly, Reaction at 15°C for 2 h, rotary evaporation to dryness under reduced pressure, extraction with dichloromethane, washing with brine, drying over anhydrous sodium sulfate, rotary evaporation to dryness under reduced pressure, the obtained crude product was recrystallized from a mixed solvent of ethyl acetate-petroleum ether to obtain Intermediate II, off-white to solid (130.0 g), yield 95%.
[0083] B) Preparation of Intermediate III
[0084] {2-[3-(1-methyl-5-(tritylamino)-1H-pyrazol-4-yl)ureido]ethyl}carbamate tert-butyl ester (155.0g, 0.29mol) In N,N-dimethylformamide (2000mL), add hexamethyldisilazi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com