Injectable composite carrier and composition with slow and controlled release drug effect and preparation method
A compound carrier and controlled-release drug technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, drug delivery, etc., can solve the problem that the slow-release injections cannot be safe, long-acting, and easy to obtain. Requirements, unable to load macromolecular protein drugs, and unable to be used repeatedly for a long time, to achieve the effects of no toxic side effects, significant clinical value, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of the composition containing recombinant human growth hormone
[0052] Compositions containing polymers and adjuvants and recombinant human growth hormone (hereinafter referred to as growth hormone) were prepared in various formulation compositions listed in Table 1. First, the polymer and the adjuvant are mixed uniformly at 80°C ± 2°C to prepare an injectable composite carrier, and then the recombinant human growth hormone is added at a reduced temperature, and the mixture is mixed until homogeneous to obtain the composition. The resulting composition was translucent liquid in appearance.
[0053] Table 1
[0054]
[0055]
Embodiment 2
[0056] Example 2 In Vitro Release of Compositions Containing Recombinant Human Growth Hormone
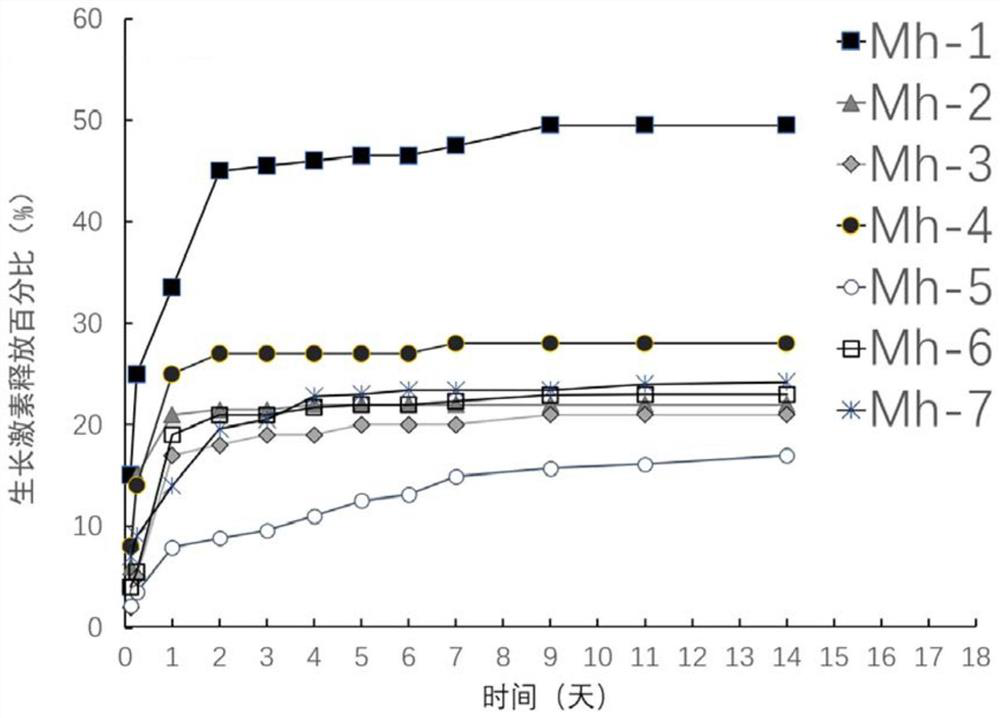
[0057] Put 0.4 grams of the seven compositions containing recombinant human growth hormone prepared in Example 1 into different tubes containing 10 mL of phosphate buffered saline (PBS), and shake them at 37°C for in vitro experiments. Release Research. Samples were taken at 3h, 6h, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 9 days, 11 days, 14 days, and fresh PBS was completely replaced. The protein content in the release solution at each time period was analyzed and determined by the BCA protein detection kit, and the cumulative release curve was drawn. The results are shown in figure 1 middle. It can be seen that the composition containing recombinant human growth hormone can stably encapsulate and release the drug for a long time under in vitro release conditions.
Embodiment 3
[0058] Example 3 In vivo release of compositions containing recombinant human growth hormone
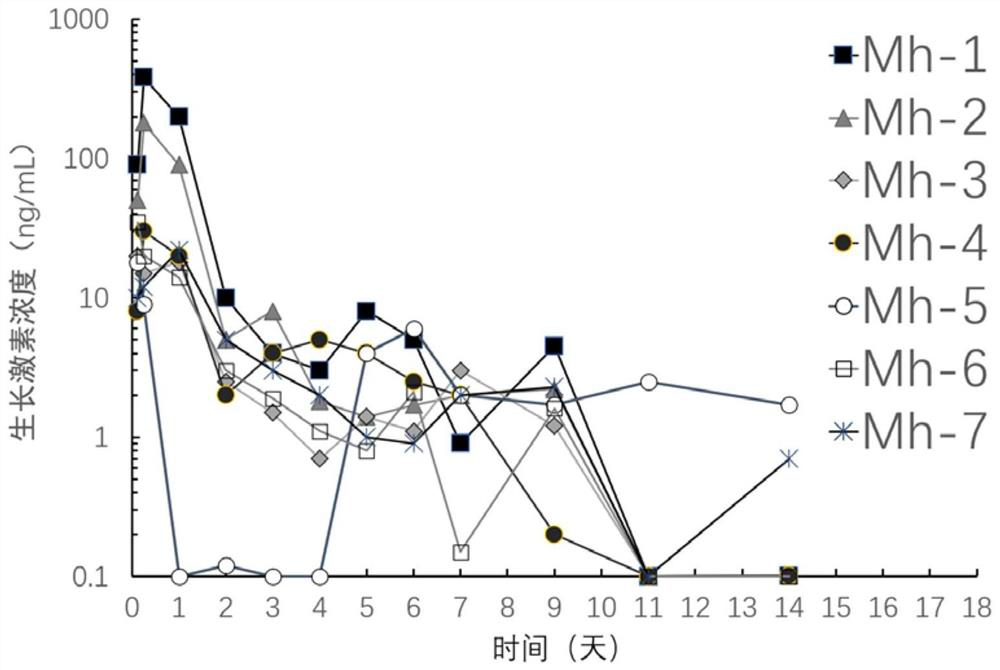
[0059] The experimental female SD rats were randomly divided into seven groups, and the rats in each group were subcutaneously injected with 0.4 g of the seven recombinant human growth hormone-containing compositions prepared in Example 1 respectively. Blood samples were taken at 3h, 6h, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 9 days, 11 days and 14 days, and the supernatant was collected by centrifugation. The content of growth hormone in the blood samples of rats at various time periods was analyzed and determined by the recombinant human growth hormone detection kit, and the blank blood samples of rats without the composition were used as the control to draw the blood drug concentration curve. The results are shown in figure 2 middle. It can be seen that the composition containing recombinant human growth hormone can maintain the concentration of growth hormone in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com