Method for synthesizing 4,4'-bis(9h-carbazol-9-yl)biphenyls in one step
A technology of compounds and carbazoles, applied in the field of organic synthesis, can solve the problems of slow post-processing, complicated steps, long time consumption, etc., and achieve the effects of low cost, low price and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
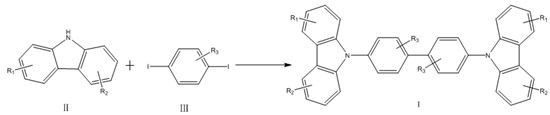
[0023] A method for synthesizing 4,4'-bis(9H-carbazol-9-yl) biphenyl compounds in one step, comprising the steps of:
[0024]
[0025] In the carbazole compounds shown in formula II in this example, R 1 and R 2 Both are hydrogen, which is carbazole; R in the 1,4-diiodobenzene compounds shown in formula III 3 is hydrogen, is 1,4-diiodobenzene;
[0026] Under air atmosphere, 167 mg (1 mmol) of carbazole, 329 mg (1 mmol) of 1,4-diiodobenzene, 224 mg (2 mmol) of potassium tert-butoxide, CuCl 2 2H 2 O 171mg (1mmol), Ag 2 CO 3 Add 331mg (1.2mmol), 8mg (0.03mmol) of 18-crown-6 and 2.5mL of DMPU into the reactor, heat to 140°C and react for 48h. After the reaction is completed, cool down, spin out the solvent DMPU, add 50mL of dichloro Methane extraction, first washed with saturated sodium carbonate solution, and then washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the solvent DMPU was spin-off, followed by column chromatogr...
Embodiment 2
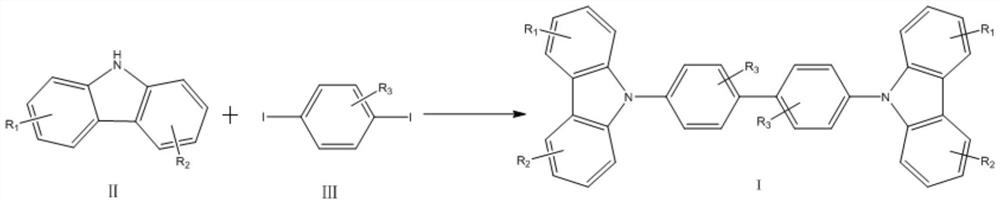
[0030] A method for synthesizing 4,4'-bis(9H-carbazol-9-yl) biphenyl compounds in one step, comprising the steps of:
[0031]
[0032] In the carbazole compounds shown in formula II in this example, R 1 and R 2 Both are methyl, respectively in the 3 and 6 positions of the carbazole skeleton, which is 3,6-dimethyl-9H-carbazole; in the 1,4-diiodobenzene compounds shown in formula III, R 3 is hydrogen, is 1,4-diiodobenzene;
[0033] Under air atmosphere, 3,6-dimethyl-9H-carbazole 195mg (1mmol), 1,4-diiodobenzene 329mg (1mmol), sodium tert-butoxide 224mg (2mmol), CuCl 2 2H 2 O 171mg (1mmol), Ag 2 CO 3 Add 331mg (1.2mmol), 18-crown ether-68mg (0.03mmol) and 2.5mL of DMPU into the reactor, heat to 130°C for 48h, after the reaction is finished, cool down, spin out the solvent DMPU, add 50mL of dichloromethane Extraction, first washed with saturated sodium carbonate solution, then washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, ...
Embodiment 3
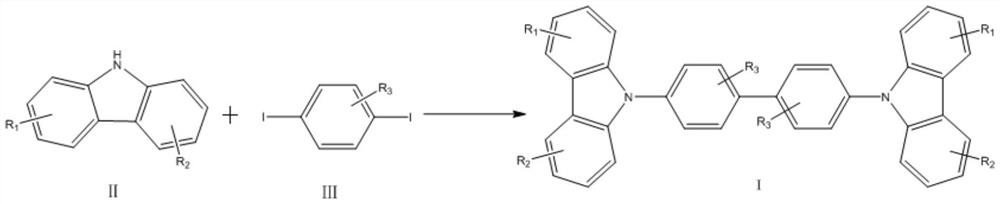
[0037] A method for synthesizing 4,4'-bis(9H-carbazol-9-yl) biphenyl compounds in one step, comprising the steps of:
[0038]
[0039] In the carbazole compounds shown in formula II in this example, R 1 and R 2 Both are bromine, respectively at the 3-position and 6-position of the carbazole skeleton, which is 3,6-dibromo-carbazole; in the 1,4-diiodobenzene compounds shown in formula III, R 3 is hydrogen, is 1,4-diiodobenzene;
[0040] Under air atmosphere, 325 mg (1 mmol) of 3,6-dibromo-carbazole, 329 mg (1 mmol) of 1,4-diiodobenzene, 224 mg (2 mmol) of potassium tert-butoxide, CuCl 2 2H 2 O 171mg (1mmol), Ag 2 CO 3 Add 331 mg (1.2 mmol), 8 mg (0.03 mmol) of 18-crown-6 and 2.5 mL of DMPU into the reactor, heat to 120 ° C for 48 hours, after the reaction is completed, cool down, spin out the solvent DMPU, add 50 mL of di Chloromethane extraction, first washed with saturated sodium carbonate solution, then washed with saturated sodium chloride solution, dried over anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com