Self-assembled nano-drug as well as preparation method and application thereof
A nanomedicine and self-assembly technology, applied in nanomedicine, nanotechnology, nanotechnology and other directions, can solve problems such as increasing safety risks, affecting biological functions, organ damage, etc. antitumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Preparation process of dasatinib self-assembled nanomedicine.
[0073] Dissolve 1 mg of dasatinib in 0.1 mL of dimethyl sulfoxide (DMSO) to prepare a 10 mg / mL organic solution; under ultrasonic vibration, slowly inject 0.1 mL of dasatinib into 0.9 mL of DMSO solution. In ultrapure water, the solution is mixed uniformly and self-assembles rapidly to form nano-drugs. The residual DMSO was removed by dialysis, and the obtained drug nanoparticles were further collected by centrifugation or lyophilization; washed with PBS, and finally uniformly dispersed in 1 mL of PBS to obtain dasatinib self-assembled nanomedicine.
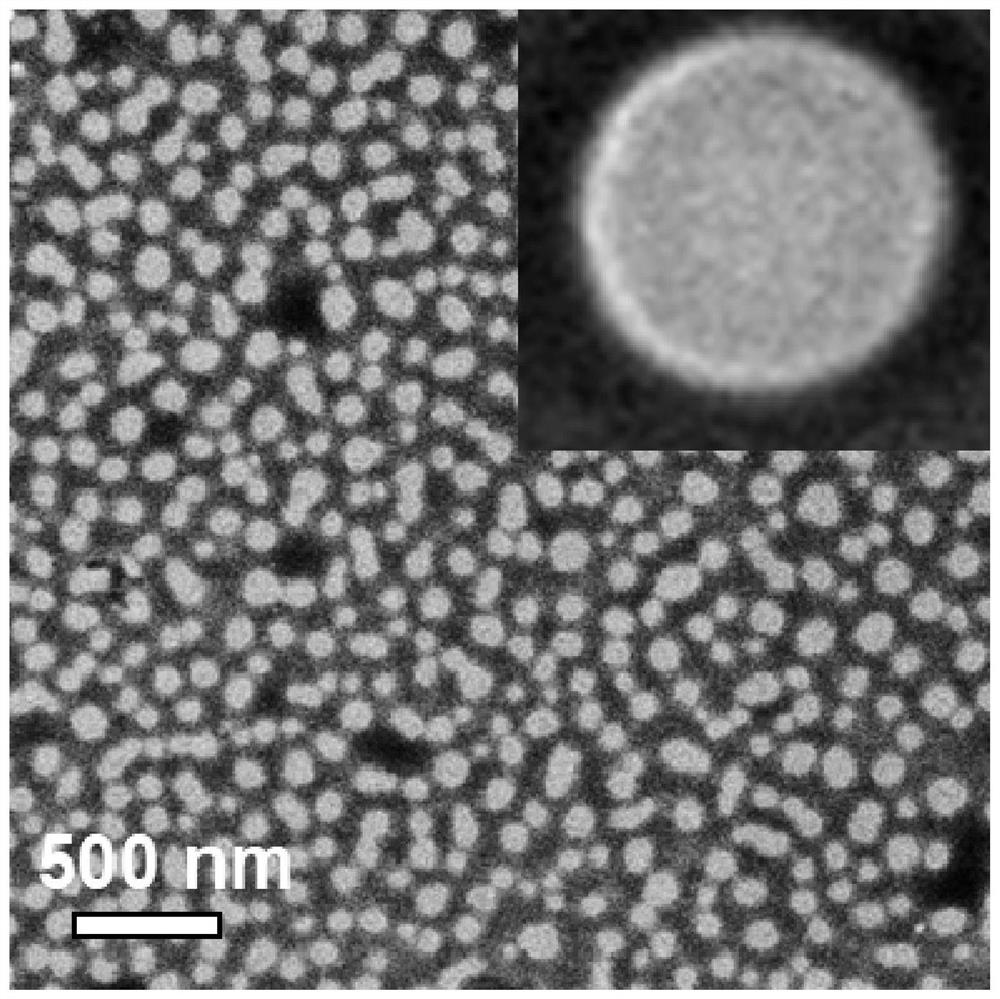
[0074] figure 2 This is the scanning electron microscope image of the self-assembled nanomedicine of dasatinib prepared in Example 1 dispersed in PBS, which intuitively shows regular spherical morphology, uniform particle size and high dispersibility.
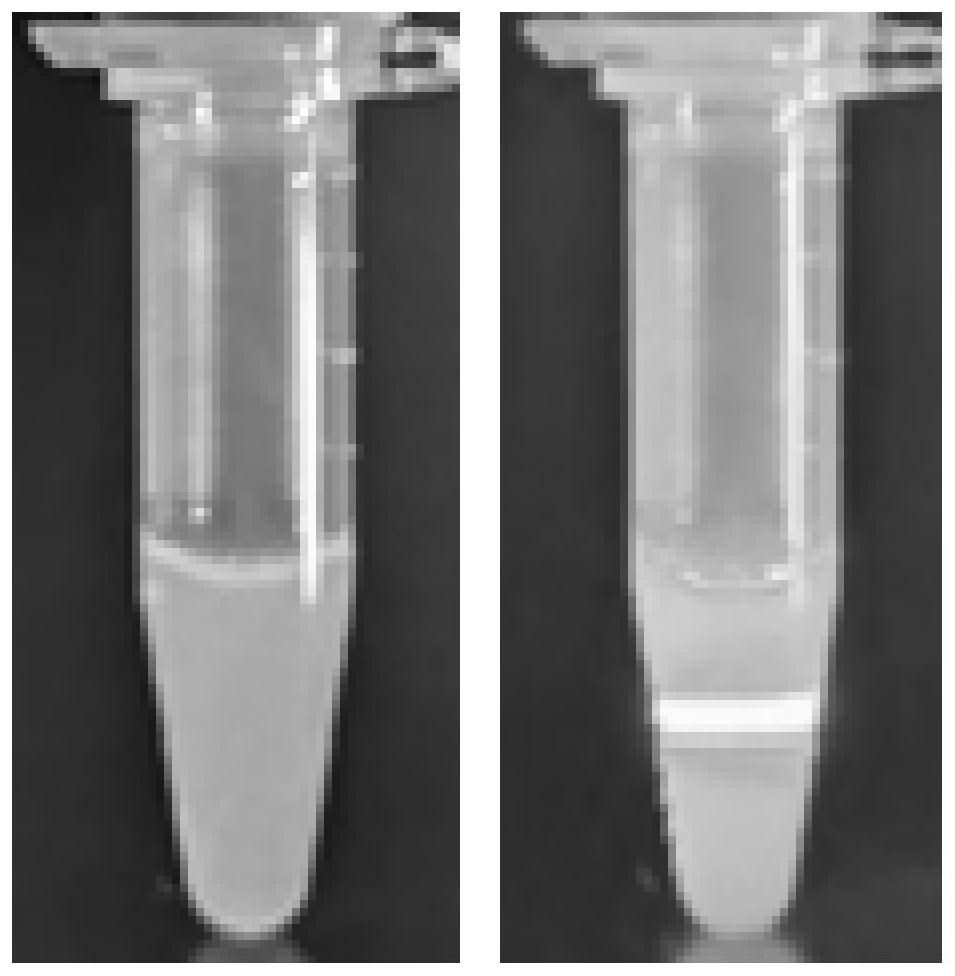
[0075] image 3 This is a photo of the dasatinib self-assembled nano-drug dispersion prepared ...
Embodiment 2
[0078] Example 2 The formation and preparation process of cabazitaxel and dasatinib spontaneously forming spherical ordered structures.
[0079] Dissolve 2 mg of cabazitaxel in 0.1 mL DMSO to prepare a first solution of 20 mg / mL; dissolve 2 mg of dasatinib in 0.1 mL of DMSO to prepare a second solution of 20 mg / mL; mix the first solution with the first solution. The two solutions were mixed evenly by volume 1:1. Under ultrasonic vibration, 0.2 mL of DMSO solution mixed with the two drugs was slowly poured into 1.8 mL of ultrapure water, so that the solution was evenly mixed and self-assembled rapidly to form nano-drugs; centrifugation Collected (50000g, 30min) to obtain the precipitate of drug nanoparticles, washed with PBS, and finally uniformly dispersed in 2 mL of PBS to obtain cabazitaxel-dasatinib co-assembled nanomedicine.
[0080] Image 6 This is the scanning electron microscope image of the cabazitaxel-dasatinib co-assembled nanomedicine synthesized in Example 2, whi...
Embodiment 3
[0083] Example 3 Preparation process of govatinib self-assembled nanomedicine.
[0084]Dissolve 1 mg of govatinib in 0.1 mL of DMSO to prepare a 10 mg / mL organic solution; under ultrasonic vibration, slowly inject 0.1 mL of govatinib in DMSO into 0.9 mL of ultrapure water to mix the solutions Uniform and rapid self-assembly occurs to form nanomedicines. The residual DMSO was removed by dialysis, and the obtained drug nanoparticles were further collected by centrifugation or freeze-drying; washed with PBS, and finally uniformly dispersed in 1 mL of PBS to obtain govatinib self-assembled nano-drug dispersion.
[0085] Figure 9 This is a photo of the self-assembled nano-drug dispersion of govatinib in Example 2. A clear and transparent solution can be observed, and the solution is left standing for 6 hours without precipitation.
[0086] Figure 10 It is the particle size distribution diagram of the govatinib self-assembled nanomedicine prepared in Example 2. It can be seen t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com