Albumin-bound hypoxia-oxidation dual-responsive composite nanoparticles, preparation method and use
A technology of albumin-binding and nanoparticle, applied in the field of pharmaceutical preparations, can solve problems such as accelerating blood clearance and reducing long-term circulation effects, and achieve the effects of reducing toxicity, uniform particle size, and improving circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
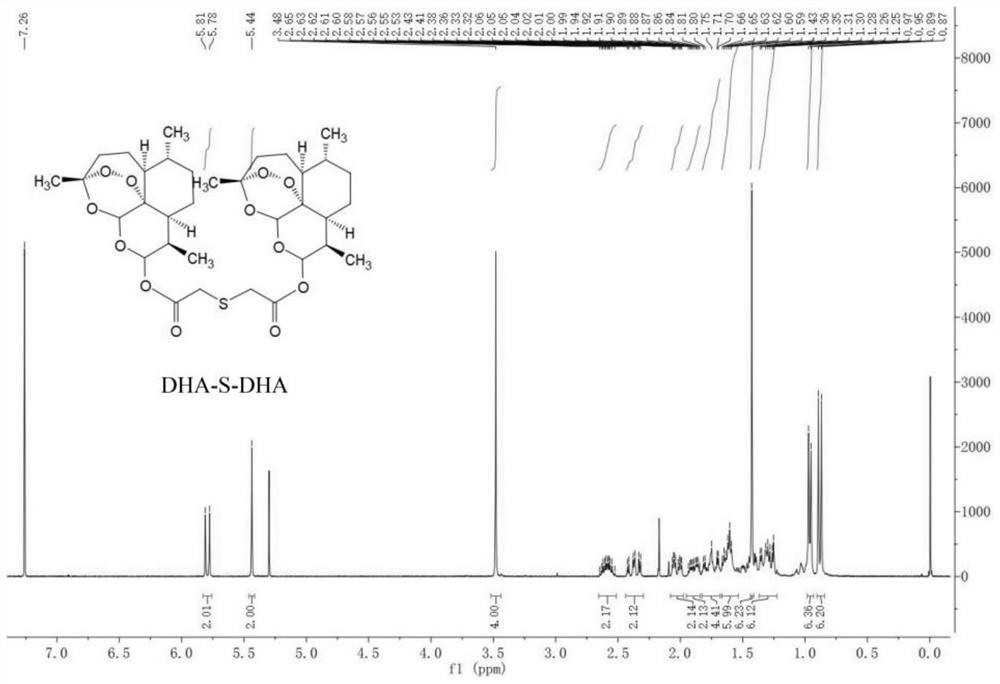
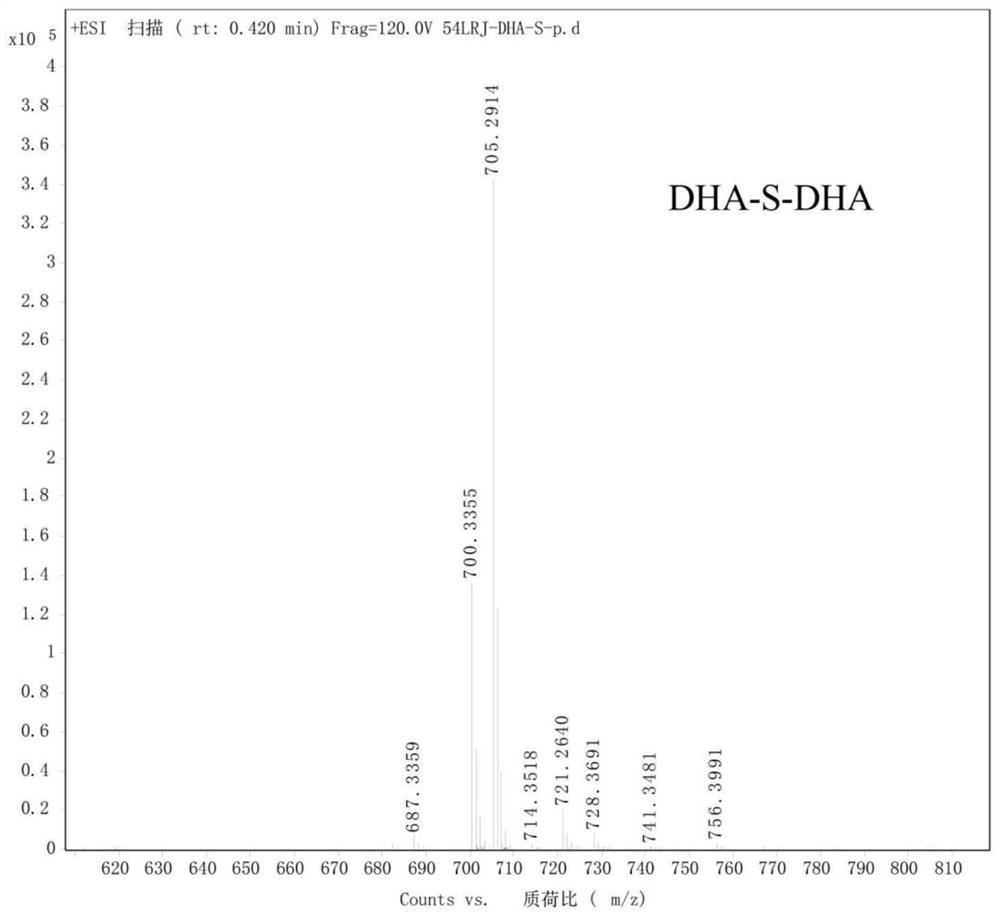
[0060] Synthesis of Dihydroartemisinin Dimer Linked by Monosulfide Bond (DHA-S-DHA)
[0061] Weigh dihydroartemisinin (227.48 mg, 0.80 mmol) and dimethylaminopyridine (9.77 mg, 0.08 mmol) and dissolve them in anhydrous dichloromethane. Glycolic anhydride (126.85 mg, 0.96 mmol) was added dropwise to the above solution and the reaction was terminated after 4 h. The above reaction product was directly put into the next step, and 1-hydroxybenzotriazole (181.60 mg, 1.34 mmol) and N,N-diisopropylethylamine (352.02 μL, 2.13 mmol) were added, and stirred for 30 min under ice bath. , 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (204.16 mg, 1.06 mmol) was added to it, and stirring was continued for 1 h under ice bath. Subsequently, dihydroartemisinin (154.68 mg, 0.54 mmol) was dissolved in dichloromethane, added dropwise, reacted under an ice bath for 1 h, and then transferred to room temperature to continue the reaction overnight. After the reaction, it was washed thre...
Embodiment 2
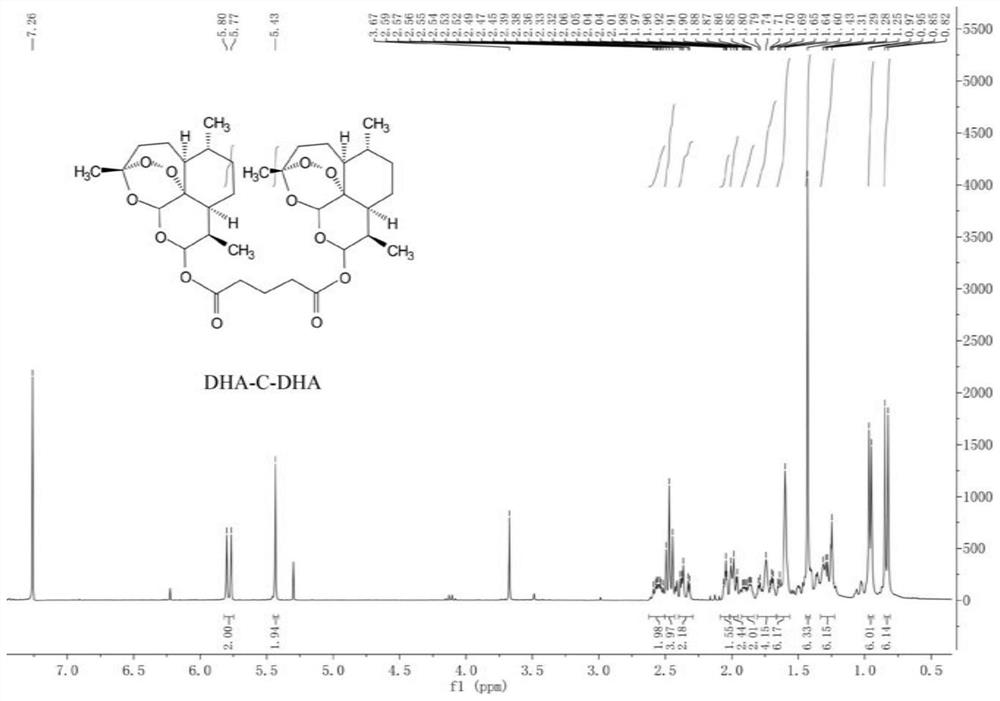
[0064] Synthesis of Carbon Single Bonded Dihydroartemisinin Dimers (DHA-C-DHA)
[0065] Weigh dihydroartemisinin (227.48 mg, 0.80 mmol) and dimethylaminopyridine (9.77 mg, 0.08 mmol) and dissolve them in anhydrous dichloromethane. Acid anhydride (109.54 mg, 0.96 mmol) was added dropwise to the above solution, and the reaction was terminated after 4 h. The above reaction product was directly put into the next step, and 1-hydroxybenzotriazole (181.60 mg, 1.34 mmol) and N,N-diisopropylethylamine (352.02 μL, 2.13 mmol) were added, and stirred for 30 min under ice bath. , 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (204.16 mg, 1.06 mmol) was added to it, and stirring was continued for 1 h under ice bath. Subsequently, dihydroartemisinin (154.68 mg, 0.54 mmol) was dissolved in dichloromethane, added dropwise, reacted under an ice bath for 1 h, and then transferred to room temperature to continue the reaction overnight. After the reaction, it was washed three times ...
Embodiment 3
[0068] Synthesis of Polymer CMCTS-MAL&NI with Carboxymethyl Chitosan as Core
[0069] 2-Nitroimidazole (0.50 g, 4.43 mmol), ethyl 6-bromohexanoate (1.04 g, 4.65 mmol) and potassium carbonate (4.90 g, 35.40 mmol)) were dissolved in acetonitrile and heated at 60°C for 6 days. After the reaction is over, by adding an appropriate amount of K 2 CO 3 The pH of the resulting solution was adjusted to 7-8. The solvent was then evaporated to dryness in vacuo, appropriate amounts of ethyl acetate and water were added, and the two phases were separated by extraction. The organic phase was washed three times with water, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain ethyl-(2-nitroimidazolyl)hexanoic acid ethyl ester. Then, ethyl-(2-nitroimidazolyl)hexanoic acid ethyl ester (1.13 g, 4.43 mmol) was placed in concentrated hydrochloric acid and stirred vigorously at room temperature overnight. The solvent was evaporated to dryness in vacuo the next day to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com