COVID-19 vaccine and preparation method and application thereof
A COVID-19 and vaccine technology, applied in the field of COVID-19 vaccine and its preparation, can solve the problems of complex preparation process, difficult preparation, and low effective antigen amount, and achieve the advantages of wide source of raw materials, low preparation cost and short preparation cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
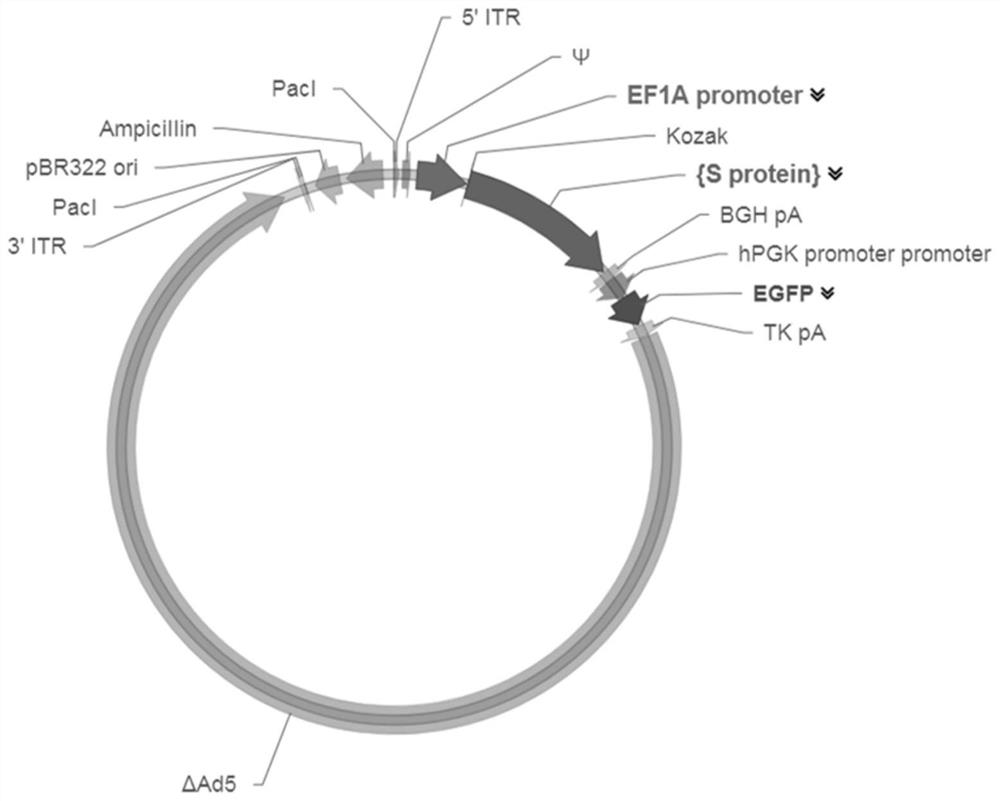
[0045] Embodiment 1 constructs the AdV5 adenoviral vector that overexpresses the SARS-CoV-2S protein coding gene
[0046] In this example, molecular cloning technology was first used to obtain the plasmid vector pUC57-S that continuously expresses the SARS-CoV-2 S protein molecule; then PCR was performed on the pUC57-S plasmid to obtain the S protein coding gene (SEQ ID NO: 2), and the homologous Recombinantly connected to the AdV5 adenovirus vector (containing GFP gene) between the Kozak and BGH pA sites, constructed as follows figure 1 The AdV5-S vector shown, the map is as follows figure 2 shown.
Embodiment 2
[0047] Embodiment 2 SARS-CoV-2 pseudovirus packaging
[0048] Cultivate 293T cells in a 10cm culture dish, the medium is DMEM high glucose medium + 10% FBS (fetal bovine serum) + 1% double antibody (100 × penicillin-streptomycin mixed solution); 293T cells in the culture dish When the density reaches 80%, replace the medium with DMEM high glucose medium + 1% FBS + 1% double antibody;
[0049] After 2 hours, prepare the transfection reagent, put 500 μL opti-DMEM into a 15 mL centrifuge tube, add 7.2 μL PEI (linear polyethyleneimine) with a concentration of 10 μg / μL, mix slightly, and let stand for 5 minutes; take 500 μL opti-DMEM - DMEM into a 1.5mL centrifuge tube, take SARS-CoV-2S protein recombinant adenovirus vector 9μg, pMD2.G helper plasmid 3μg and psPAX 12μg, add to the centrifuge tube, mix well, add to the transfection reagent, mix upside down Evenly, let stand for 20min;
[0050] Add all the above mixture to 293T cells, and after incubation for 6 hours, replace with ...
Embodiment 3
[0053] Example 3 Construction of T cells expressing SARS-CoV-2 S protein
[0054] T cell acquisition and sorting: C57 / BL6 mice were dissected in a sterile environment after decapitation, and the complete spleen was isolated; the spleen was ground with a syringe, and filtered through a sieve to form a single-cell suspension; use a mouse Pan T cell isolation kit for T cell sorting, the target is to obtain live T cells ≥ 4×10 7 , survival rate ≥ 70%;
[0055] T cell activation: 24-well plates pre-coated with anti-CD3 / CD28 antibodies were used for T cell activation, and 1×10 7 Resuspend mouse spleen T cells in 2 mL of 1640 medium containing 10% FBS and 300 IU mouse IL-2, add them to a 24-well plate, and place at 37°C, 5% CO 2 Activate in the incubator for 48±3 hours;
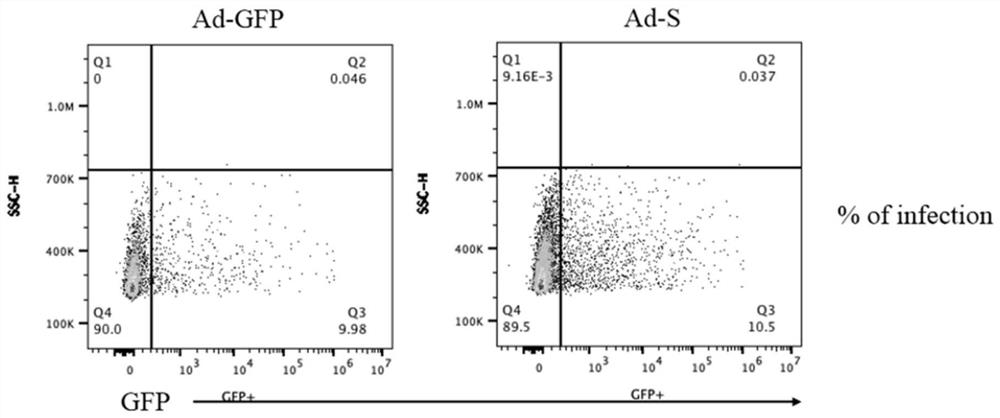
[0056] Adenovirus transduction: The activated mouse T cells were collected by centrifugation and resuspended at 1×10 7 The proportion of MOI=200 virus solution was added to each cell / well for AdV transduction, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com