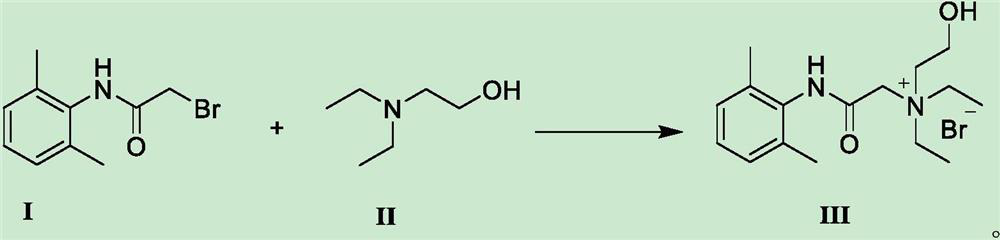
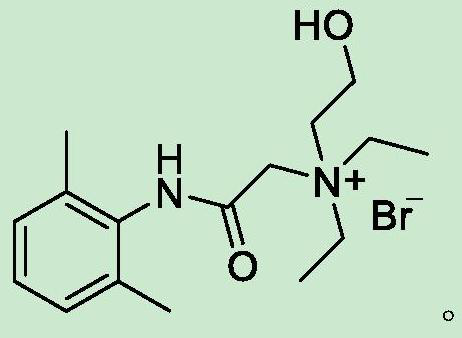
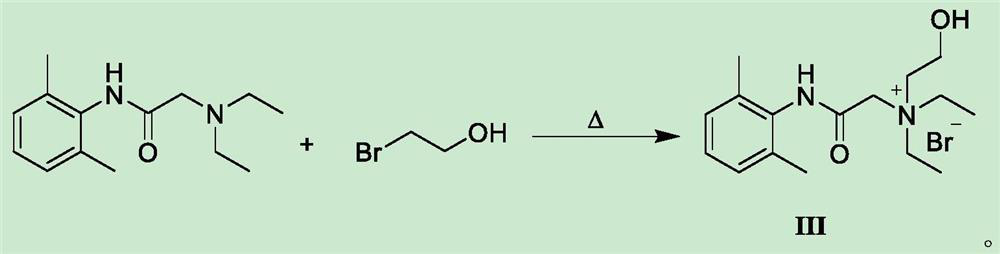
Preparation method of N-diethylaminoacetyl-2,6-dimethylaniline quaternary ammonium salt
A technology of diethylaminoacetyl and dimethylaniline, applied in the field of pharmaceutical synthesis, can solve the problems of unstable raw materials, low purity, low yield and the like, and achieves a complete reaction, high yield and purity, and economical and environmentally friendly yields. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 24.2g of 2-bromo-N-(2,6-dimethylphenyl)acetamide and add it to 100ml of acetonitrile solution, add 21.1g of diethylaminoethanol, heat to 75-80°C and stir to react, TLC detects that the reaction is complete After cooling down to room temperature to obtain solid QX-314-OH, add the obtained crude QX-314-OH to 50ml of anhydrous ether and heat to dissolve, then cool down to 20°C, keep stirring and crystallize for 3h, filter with suction, and vacuum-dry the obtained filter cake at 45°C The yield of QX-314-OH was 73.4%, and the HPLC purity was 99.56%.
Embodiment 2
[0029] Weigh 24.2g of 2-bromo-N-(2,6-dimethylphenyl)acetamide and add it to 100ml of toluene solution, add 21.1g of diethylaminoethanol, heat to 80-85°C and stir to react, TLC detects that the reaction is complete After cooling down to room temperature to obtain solid QX-314-OH, add the obtained crude QX-314-OH to 60ml of methanol for heating and dissolving, then lower the temperature to 10°C, keep stirring and crystallize for 2 hours, filter with suction, and dry the obtained filter cake under vacuum at 45°C to obtain QX -314-OH, the yield is 71.7%, and the HPLC purity is 99.48%.
Embodiment 3
[0031] Weigh 24.2g of 2-bromo-N-(2,6-dimethylphenyl)acetamide and add it to 100ml of acetonitrile solution, add 16.4g (0.14mol) of diethylaminoethanol, heat to 60-65°C and stir to react. After TLC detection, the reaction was lowered to room temperature to obtain solid QX-314-OH, and the obtained crude QX-314-OH was added to 50ml of anhydrous ether for heating and dissolving, then cooled to 25°C for 3 hours with stirring and crystallization, and filtered with suction to obtain the filter cake Vacuum drying at 45°C gave QX-314-OH with a yield of 70.5% and an HPLC purity of 99.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com