Sustained-release pellet containing huperzine A microcapsules and preparation method thereof
A technology of huperzine A and sustained-release pellets, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, which can solve the problems of inconvenient use for elderly patients and achieve good sustained release Effect, Enhanced Bioavailability, Enhanced Effect of Sustained Release Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
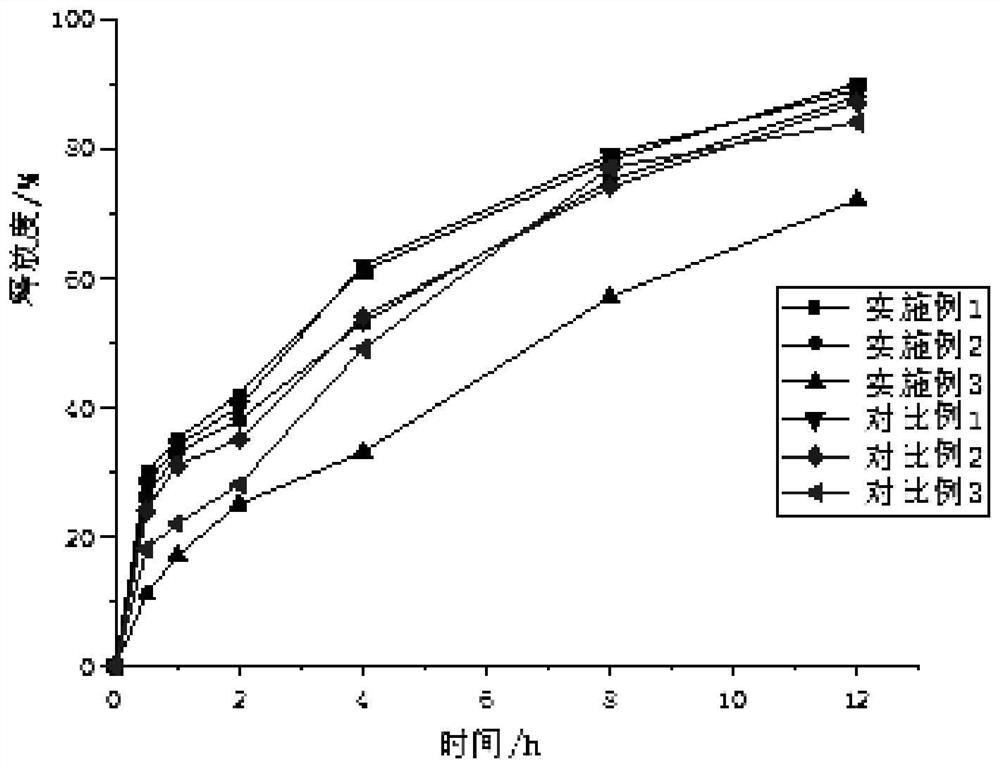
Image
Examples
Embodiment 1
[0026] A sustained-release pellet containing huperzine A microcapsules and a preparation method thereof, the formulation of which is shown in Table 1.
[0027] Table 1
[0028] components effect Dosage Huperzine A Microcapsules main ingredient 0.1mg HPMC K4M Adhesive 6mg Poloxamer 407 thickener 0.4mg microcrystalline cellulose Pilling accessories 20mg lactose Pilling accessories 14mg Mannitol Pilling accessories 8mg Sodium carboxymethyl cellulose Pilling accessories 12mg purified water solvent 60mL
[0029] Preparation method: ① Preparation of huperzine A microcapsules: Add 50 mg of huperzine A raw material into 200 mL of 0.5% sodium alginate aqueous solution, stir well, then drop 100 mL of 1% chitosan-acetic acid aqueous solution into the above-mentioned In the turbid liquid, the huperzine A microcapsule emulsion is prepared by complex coacervation, and then the huperzine A microcapsule ...
Embodiment 2
[0037] A sustained-release pellet containing huperzine A microcapsules and a preparation method thereof, the formulation of which is shown in Table 3.
[0038] table 3
[0039] components effect Dosage Huperzine A Microcapsules main ingredient 0.2mg HPMC K4M Adhesive 8mg Poloxamer 407 thickener 0.6mg polyethylene glycol 6000 plasticizer 2mg microcrystalline cellulose Pilling accessories 20mg lactose Pilling accessories 16mg Mannitol Pilling accessories 8mg Sodium carboxymethyl cellulose Pilling accessories 15mg purified water solvent 70mL
[0040] Preparation method: ① Preparation of huperzine A microcapsules: Add 50 mg of huperzine A raw material into 200 mL of 0.5% sodium alginate aqueous solution, stir well, then drop 100 mL of 1% chitosan-acetic acid aqueous solution into the above-mentioned In the turbid liquid, the huperzine A microcapsule emulsion is prepared by complex co...
Embodiment 3
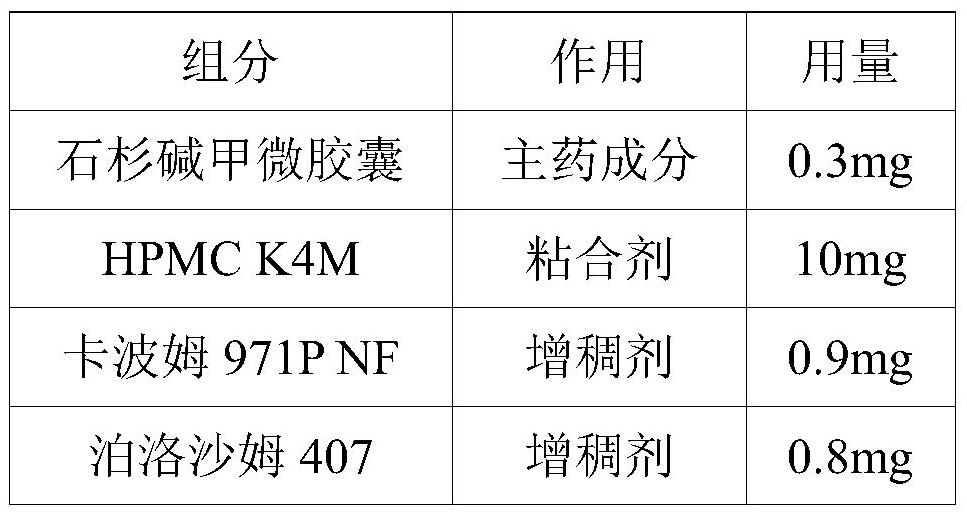
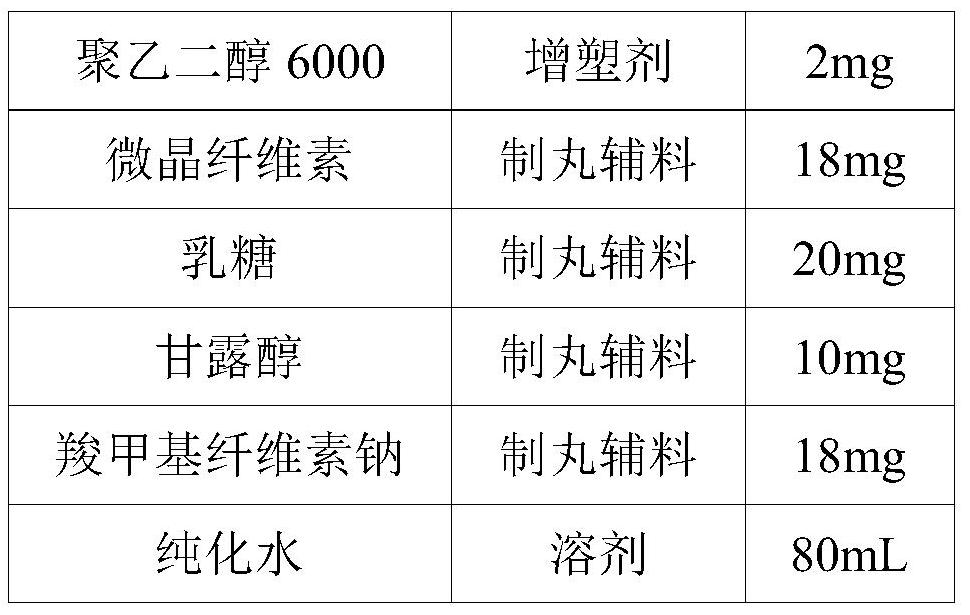
[0048] A sustained-release pellet containing huperzine A microcapsules and a preparation method thereof, the formulation of which is shown in Table 5.
[0049] table 5
[0050] components effect Dosage Huperzine A Microcapsules main ingredient 0.3mg HPMC K4M Adhesive 10mg Carbomer 971P NF thickener 0.9mg Poloxamer 407 thickener 0.8mg polyethylene glycol 6000 plasticizer 2mg microcrystalline cellulose Pilling accessories 18mg lactose Pilling accessories 20mg Mannitol Pilling accessories 10mg Sodium carboxymethyl cellulose Pilling accessories 18mg purified water solvent 80mL
[0051] Preparation method: dissolve the binder (HPMC K4M) and additives (poloxamer 407, polyethylene glycol 6000 and carbomer 971P NF) in pure water, and the resulting solution is used as a wetting agent; then the wetting agent It is uniformly mixed with huperzine A microcapsule powder and pelletizin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com