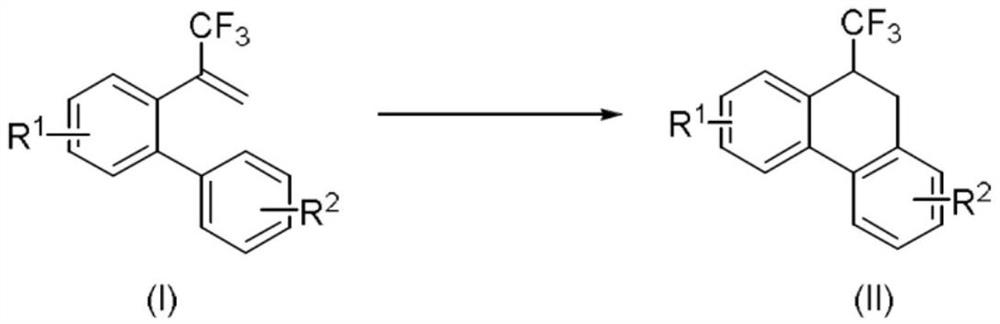
Method for synthesizing 9-trifluoromethyl-9,10-dihydrophenanthrene compound through copper photocatalysis
A technology of trifluoromethyl and 10-, which is applied in the field of synthesizing 9-trifluoromethyl-9,10-dihydrophenanthrene compounds, can solve the problems of unfavorable industrial production and large environmental impact, and achieve substrate adaptation Good performance, low operation risk, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
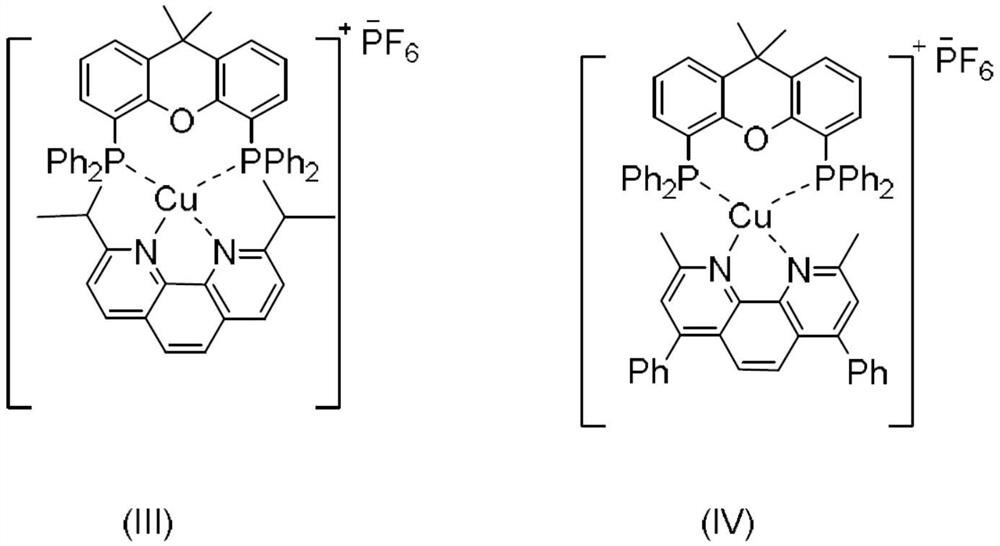
[0037] Add α-trifluoromethyl-2-phenylstyrene (0.3mmol, 0.0744g), photosensitizer (III) (0.015mmol, 0.0167g), potassium phosphate (0.45mmol, 0.0954g) to 15mL lock reaction In the tube, 3 mL of tetrahydrofuran was added as a solvent. Then, under the irradiation of 15w Blue LED, react at 25°C and nitrogen atmosphere for 24h. After the reaction, add two spoons (0.5g) of column chromatography silica gel (100-200 mesh) to the reaction solution, and pass the decompression The solvent was distilled off, and the pure product shown in the structural formula was obtained by column chromatography (using petroleum ether / ethyl acetate=20:1 as eluent). The material was a yellow liquid, 77% yield.
[0038] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ7.84(d, J=7Hz, 1H), 7.77(d, J=8Hz, 1H), 7.43(td, J 1 =7.6Hz,J 2 =1.4Hz,1H),7.37(d,J=7.4Hz,1H),7.34–7.28(m,2H),7.28–7.22(m,2H),3.61-3.54(m,1H),3.27(dd, J 1 =16.2,J 2 =6.5Hz,1H),3.20(dd,J 1 =16.2Hz,J 2 =3.2Hz,1H). 13 C NM...
Embodiment 2
[0040]
[0041] Add α-trifluoromethyl-2-phenylstyrene (0.3mmol, 0.0744g), photosensitizer (IV) (0.015mmol, 0.0172g), potassium phosphate (0.45mmol, 0.0954g) to 15mL lock reaction In the tube, 3 mL of tetrahydrofuran was added as a solvent. Then, under the irradiation of 15w Blue LED, under the condition of 25 ℃, under nitrogen atmosphere, react for 24h. After the reaction, add two tablespoons of column chromatography silica gel (100-200 mesh) to the reaction liquid, and remove the solvent by distillation under reduced pressure. The pure product shown in the structural formula was obtained by column chromatography (petroleum ether / ethyl acetate = 20:1 as eluent). The material was a yellow liquid and the yield was 67%.
[0042] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ7.84(d, J=7Hz, 1H), 7.77(d, J=8Hz, 1H), 7.43(td, J 1 =7.6Hz,J 2 =1.4Hz,1H),7.37(d,J=7.4Hz,1H),7.34–7.28(m,2H),7.28–7.22(m,2H),3.61-3.54(m,1H),3.27(dd, J 1 =16.2,J 2 =6.5Hz,1H),3.20(dd,J 1 =16.2H...
Embodiment 3
[0044]
[0045] Add α-trifluoromethyl-2-phenylstyrene (0.3mmol, 0.0744g), photosensitizer (III) (0.003mmol, 0.0034g), potassium phosphate (0.45mmol, 0.0954g) to 15mL lock reaction In the tube, 3 mL of tetrahydrofuran was added as a solvent. Then, under the irradiation of 15w Blue LED, under the condition of 25°C, under nitrogen atmosphere, react for 24h. After the reaction, add two spoonfuls of column chromatography silica gel (100-200 mesh) to the reaction solution, and remove the solvent by distillation under reduced pressure. The pure product shown in the structural formula was obtained by column chromatography (petroleum ether / ethyl acetate = 20:1 as eluent). The material was a yellow liquid, 51% yield.
[0046] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ7.84(d, J=7Hz, 1H), 7.77(d, J=8Hz, 1H), 7.43(td, J 1 =7.6Hz,J 2 =1.4Hz,1H),7.37(d,J=7.4Hz,1H),7.34–7.28(m,2H),7.28–7.22(m,2H),3.61-3.54(m,1H),3.27(dd, J 1 =16.2,J 2 =6.5Hz,1H),3.20(dd,J 1 =16.2Hz,J 2 =3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com