Graphene modified ferrate material as well as preparation method and application thereof
A technology of ferrate and graphene, which is applied in the direction of electrical components, electrochemical generators, battery electrodes, etc., to achieve the effect of improving efficiency and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention provides a kind of preparation method of the ferrate material of graphene modification in the first aspect, described method comprises the steps:
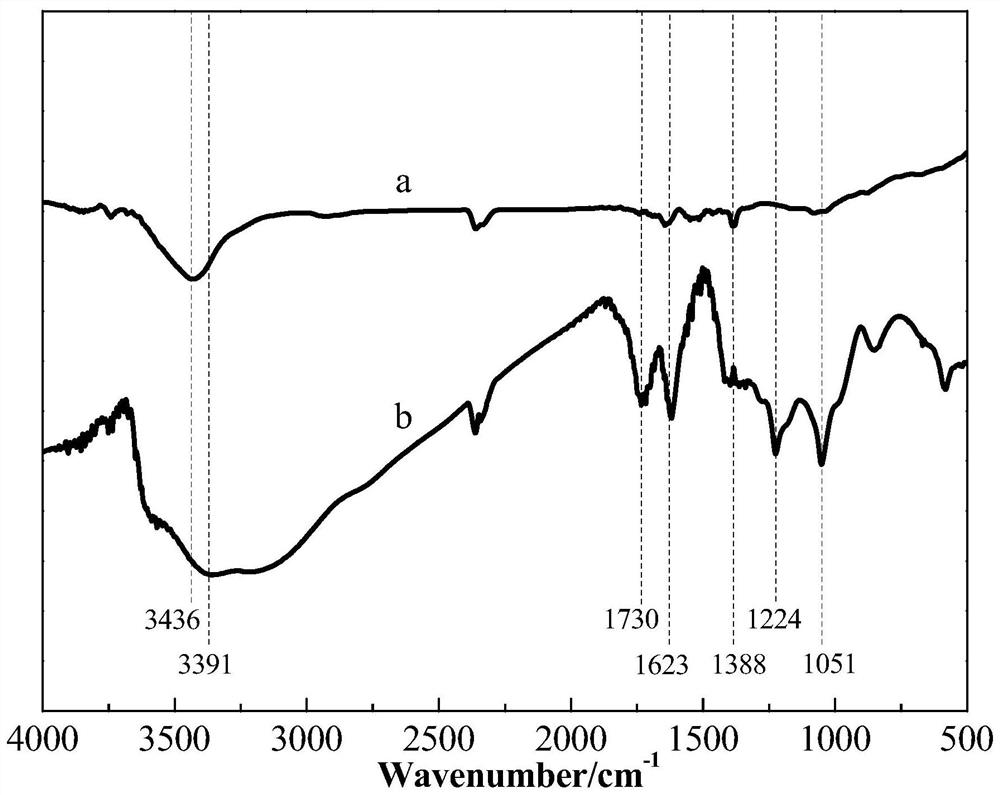
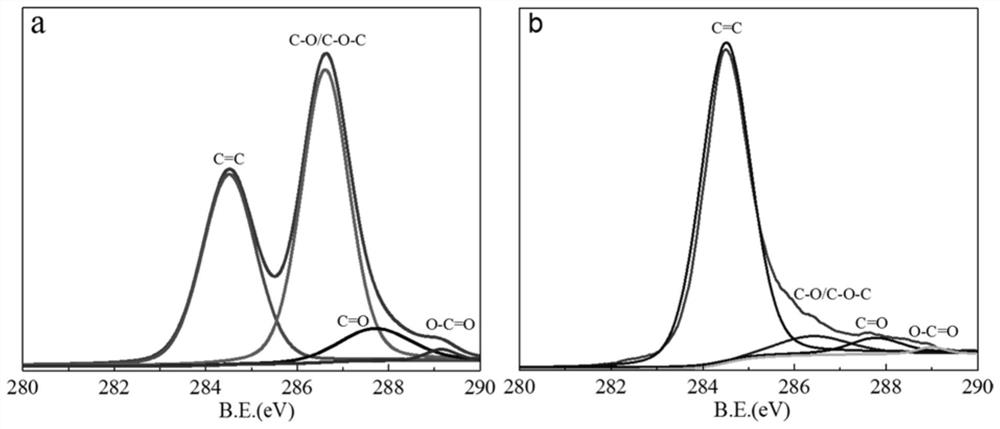
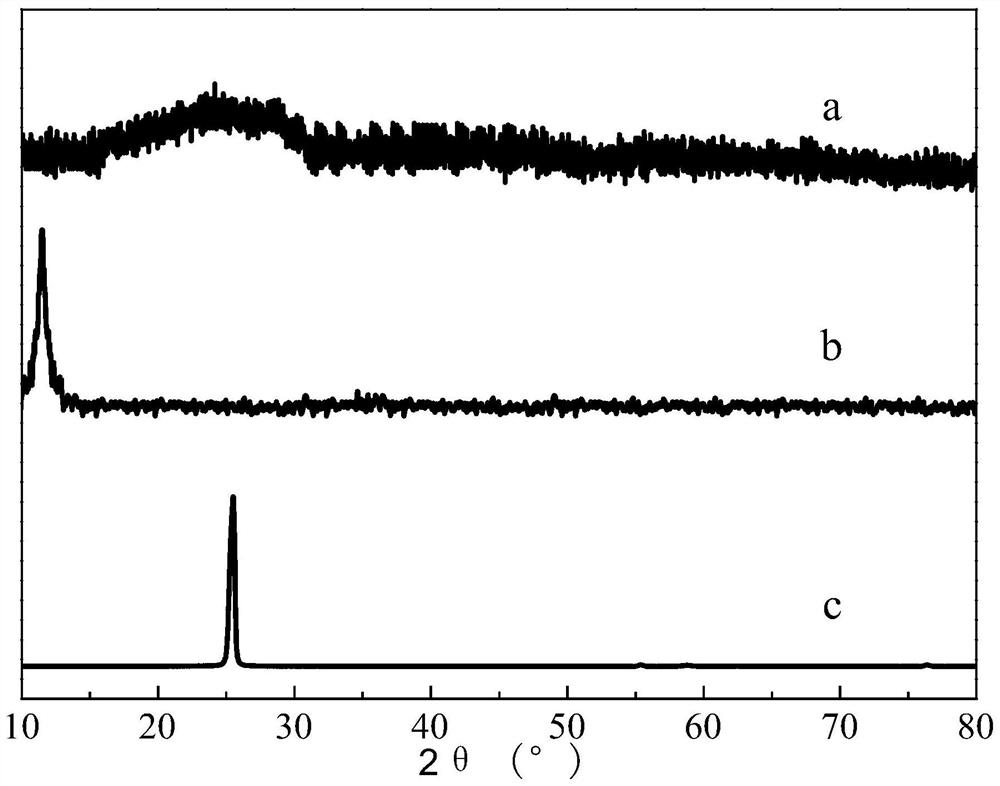
[0033] (1) prepare strongly acidic graphite oxide (pH is not more than 1 graphite oxide), wash described strongly acidic graphite oxide repeatedly with the mixed solution containing nitrogen methylpyrrolidone (NMP) and dehydrated alcohol, obtain neutral graphite oxide, Then the neutral graphite oxide is dispersed uniformly with water to obtain a graphite oxide aqueous dispersion (GO dispersion); in the present invention, graphite oxide is graphene oxide, and the graphite oxide aqueous dispersion refers to a neutral oxidized Graphite is a dispersoid, and water is a dispersion medium of a dispersion medium; in the present invention, for example, graphite powder can be used as a raw material, and the strongly acidic graphite oxide is prepared by the Hummers method; in the present invention, the neutral grap...
Embodiment 1
[0048] Embodiment 1: the preparation of the ferrate material of graphene modification
[0049] ①Add the two-system mixed solution of NMP and absolute ethanol into the strongly acidic graphite oxide according to the volume ratio of strong acidic graphite oxide:NMP:ethanol=1:2:4, and stir for 5 minutes, then stand still for 20 minutes Minutes; then vacuum filter the mixed solution that was placed for 20min, stir and disperse the obtained filter cake in 100mL distilled water, and separate the graphite oxide with weakened acidity by centrifugation (8000r / min, 15min); finally reuse NMP, absolute ethanol and deionized water treatment acid weakened graphite oxide (each time acidic graphite oxide: NMP: the volume ratio of ethanol is 1:2:4), until about its pH=7, obtains described neutral graphite oxide; The neutral graphite oxide is uniformly dispersed to obtain a graphite oxide aqueous dispersion (GO dispersion) with a mass concentration of graphite oxide of 0.15 mg / mL; wherein, the ...
Embodiment 2
[0056] Example 2: CRG coated pair K 2 FeO 4 Stability Effect Experiment
[0057] Using K in Example 1 2 FeO 4 and CRG-coated K 2 FeO 4 (3 times) experiment: three copies of K 2 FeO 4 Place them in dry air at normal temperature, in humid air environment (humid environment) and in saturated KOH solution environment respectively, and put two CRG-coated K 2 FeO 4 (3 times) respectively placed in humid air environment and saturated KOH solution environment, study K 2 FeO 4 , CRG coated type K 2 FeO 4 (3 times) the change of purity over time in different environments, the results are as follows Figure 5 shown.
[0058] Depend on Figure 5 It can be known that in dry air at room temperature, K 2 FeO 4 The purity of K decreased by only 1.1% within 60d, indicating that K 2 FeO 4 Very stable in dry air. However, K 2 FeO 4 and CRG-coated K 2 FeO 4 (3 times) After being placed in a humid environment for 60 days, the purity has dropped significantly, to 12.8% and 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com