Oxygen evolution reaction catalyst, preparation, application, electrolysis device and seawater cracking method
A technology of oxygen evolution reaction and catalyst, which is applied in the direction of electrolysis components, electrolysis process, electrodes, etc., can solve problems such as not being able to meet high current density, achieve excellent OER performance, high performance, and meet the effect of high current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present application also provides a method for preparing an epoxy reaction catalyst, including the following steps:
[0036] The foam is immersed in a metal precursor ethanol solution, and dried; the metal precursor ethanol solution is an ethanol solution of ferric chloride and nickel chloride;
[0037] Avation is added to the metal precursor ethanol solution, and the dried foam nickel is immersed therein, and the oxygenation reaction catalyst is obtained.
[0038] Specifically, a metal precursor ethanol solution can be configured, iron chloride and nickel chloride are dissolved in ethanol in ethanol. The foamed nickel is then immersed in a metal precursor ethanol solution for a certain time such as 10 to 20 minutes, and the foam can be tiled, and can be dried through room temperature can also be dried or blowing at a relatively low temperature. Do. The dry ethanol volatilization, iron chloride and nickel chloride are attached to the foam nickel.
[0039] After removing...
Embodiment 1
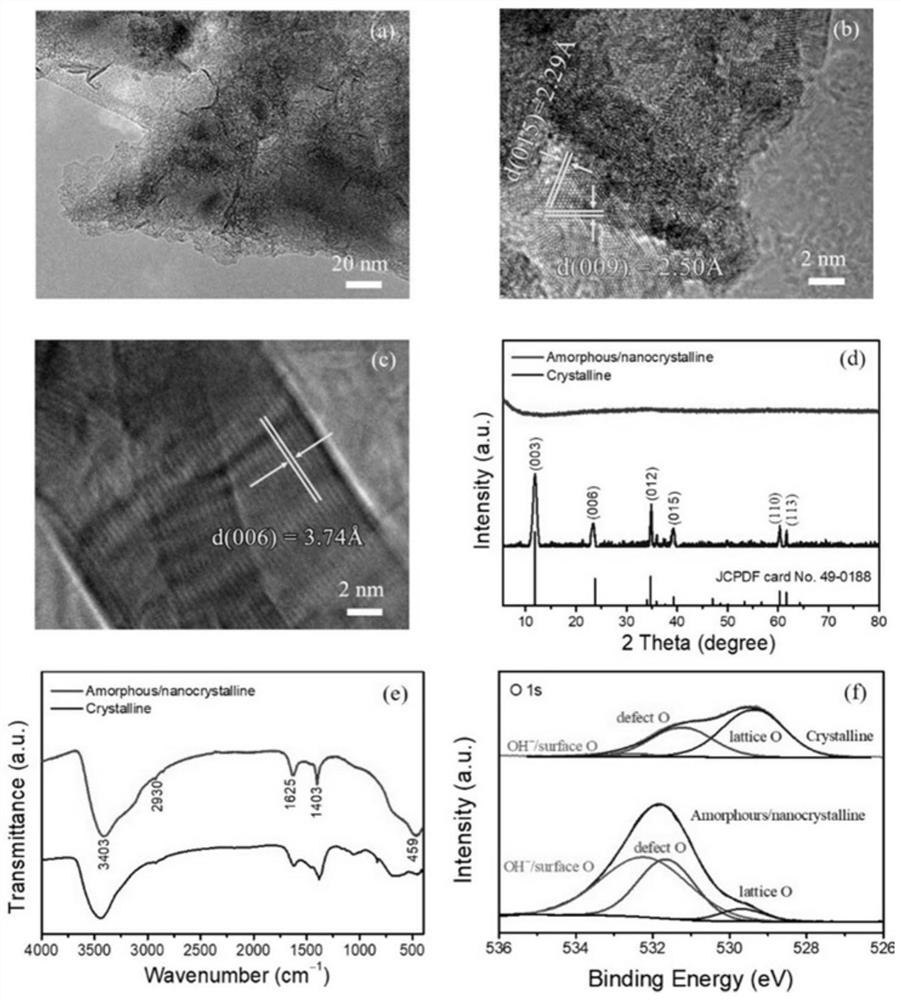
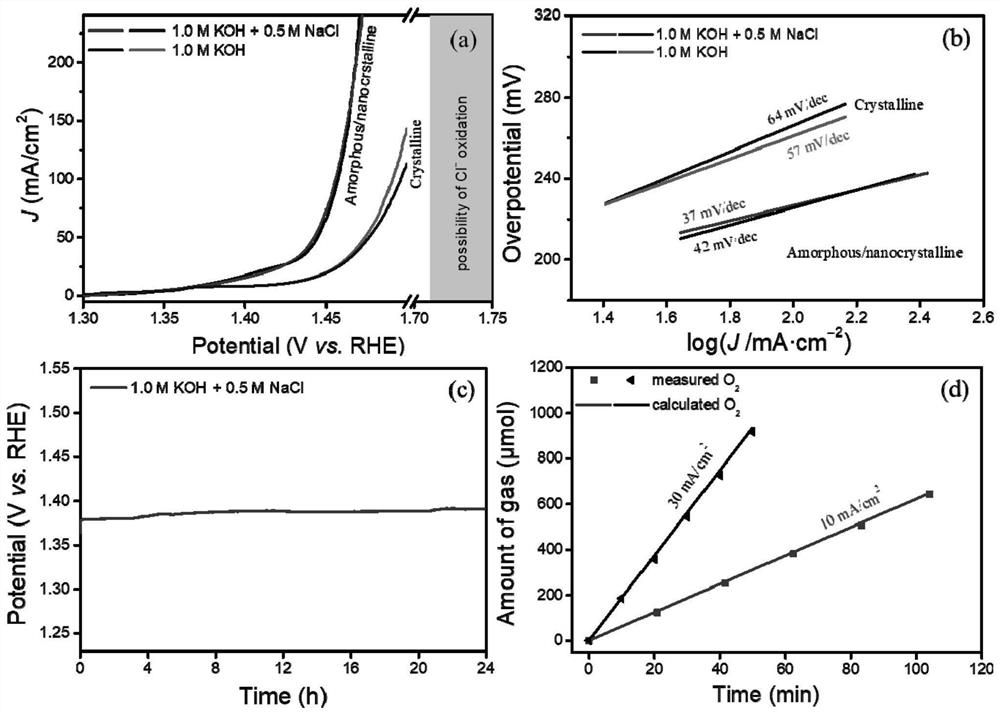
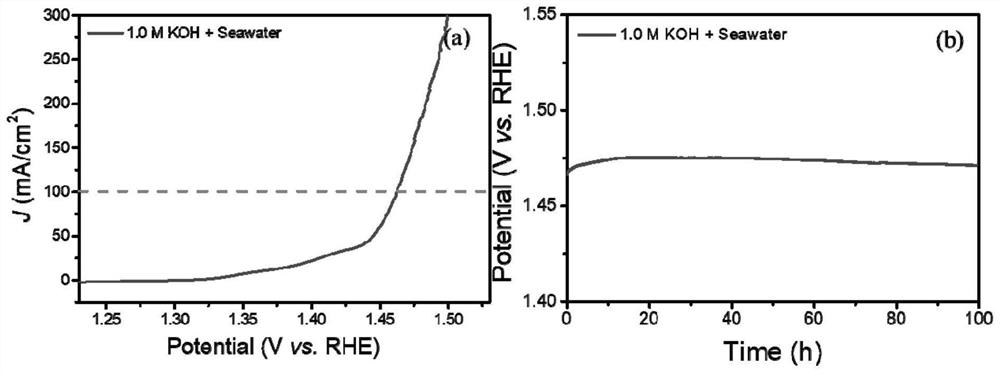
[0063] Preparation oxygen evolution reaction catalyst (amorphous / nanocrystalline and crystalline FeNiCH / NF) of
[0064] With 3.0M hydrochloric acid, 95% ethanol and deionized water three times NF ultrasonic cleaning to remove surface impurities such as nickel oxide. The one NF (20 × 10 × 1.8mm) was immersed in containing 24.4mM FeCl 3 · 6h 2 O + 2.71mM NiCl 2 · 6h 2 O 60mL ethanol solution for 15 minutes, taken out and air dried overnight. Then 0.71g NH 4 HCO 3 Was added to the ethanol solution of the metal precursor, with vigorous stirring. NF above at room temperature for 6 hours immersed in the above solution, so that the surface covered with a layer of iron-nickel hydroxycarbonate (FeNiCH). Amorphous / nanocrystalline obtained FeNiCH / NF soak for 10 to 20 minutes to remove surface impurities, and then dried in air overnight to give the amorphous / nanocrystalline and crystalline FeNiCH / NF in deionized water.
Embodiment 2
[0066] This embodiment provides an electrolysis apparatus, which electrolysis apparatus electrolytic cell comprising an anode, the electrolytic cell of Example 1 of the amorphous / nanocrystalline and crystalline FeNiCH / NF, the electrolytic cell cathode MoNi 4 / NF.
[0067] MoNi 4 / NF exemplary catalysts employed be synthesized as follows.
[0068] MoNi 4 Synthesis / NF catalyst. With 3.0M hydrochloric acid, 95% ethanol and deionized water three times NF ultrasonic cleaning to remove surface impurities such as nickel oxide. 15mL formulation containing Ni (NO 3 ) 2 · 6h 2 O (0.04M) and (NH 4 ) 6 Mo 7 O 24 · 4h 2 O (0.01M) of an aqueous solution, an aqueous solution, and NF (20 × 10 × 1.8mm) was transferred to a capacity of 40mL teflon-lined stainless steel autoclave and sealed under 150 deg.] C for 6 hours, then cooled to at room temperature. After washing with deionized water, the surface of the NF NiMoO 4 cuboid. Finally, the synthesis of NiMoO 4 In the rectangular parallelep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com