Application of dibenzyl tetrahydroisoquinoline derivative in preparation of anti-coronavirus drug
A coronavirus and application technology, applied in the field of biomedicine, can solve the problems of lack of small molecule drugs, high price, large side effects, etc., and achieve good application prospects and excellent inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
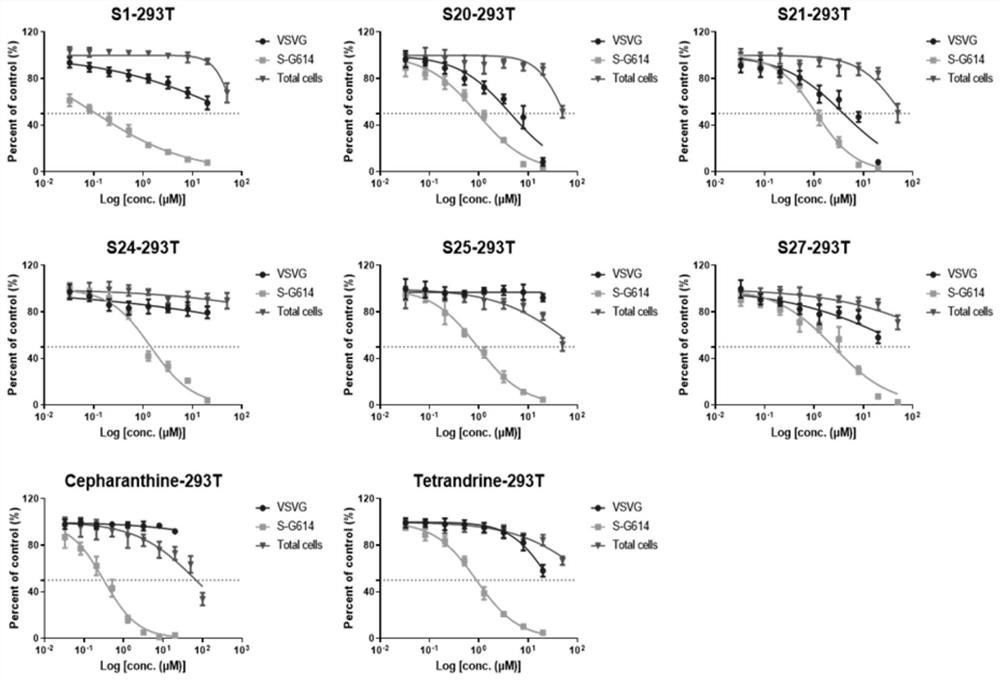
[0037] Embodiment 1: Compound is to the inhibitory activity, specificity and cytotoxicity of SARS-CoV-2 pseudovirus infection on HEK293T cells
[0038] 1. Fake virus packaging
[0039] Will 2×10 7 A HEK293T cell was seeded in a 10cm culture dish (80%-90% density),
[0040]Transfection was carried out 12 hours later (refer to the instruction manual of Lipo8000), and the transfection ratio was Opti-MEM500μl+Lipo8000 18μl+the following plasmids:
[0041] pNL4-3-Luc-R-E: VSVG=5:1 (10 μg, 2 μg);
[0042] pNL4-3-Luc-R-E: pCMV3-SARS-CoV-2.Spike (D614) = 1:1 (6 μg, 6 μg);
[0043] pNL4-3-Luc-R-E: pCMV3-SARS-CoV-2.Spike (G614) = 1:1 (6 μg, 6 μg);
[0044] pNL4-3-Luc-R-E: pCMV3-SARS-CoV.Spike=1:1 (6 μg, 6 μg);
[0045] pNL4-3-Luc-R-E:pCMV3-MERS-CoV.Spike=1:1 (6 μg, 6 μg)
[0046] The medium was changed 12 hours after transfection, and the supernatant was collected after 48 hours and 72 hours of normal culture, centrifuged for 5 minutes (4°C, 4000 rpm), and the virus supernatant wa...
Embodiment 2
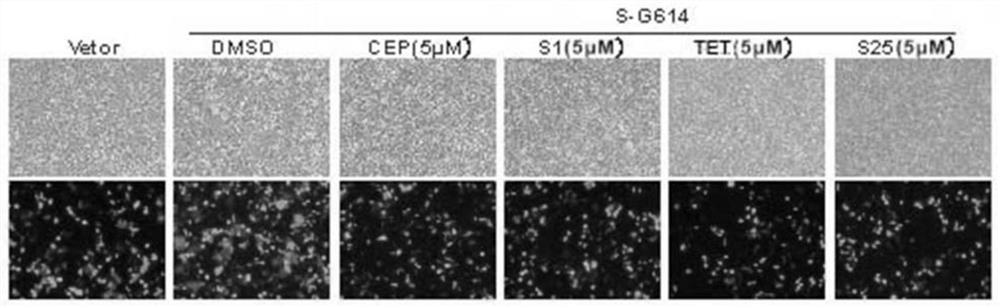
[0061] Embodiment 2: The inhibitory activity of compound on different cells to SARS-CoV-2 pseudovirus infection
[0062] 1. Inhibitory activity test of the compound to be tested against pseudovirus infection
[0063] The antiviral infection activity of the target compound on different cells was tested by luciferase activity, and stepherine and tetrandrine were used as positive controls. HEK293T, Calu-3 or A549 cells (2x10 4 cells / well) were incubated with gradient concentrations of various compounds for 1 hour, and the same amount of pseudovirus SARS-CoV-2 (S-G614) (50 μL, 3.8×10 4 copy) infection. The medium was replaced with fresh DMEM 8 h after infection. 72 hours after infection, the cells were collected and lysed with 30 μl lysis buffer (Promega), and the RLU value was measured with luciferase assay reagent (Promega) according to the product description, and the effect of the test compound on SARS-CoV-2 (S-G614) pseudovirus was calculated. Infectious median effective ...
Embodiment 3
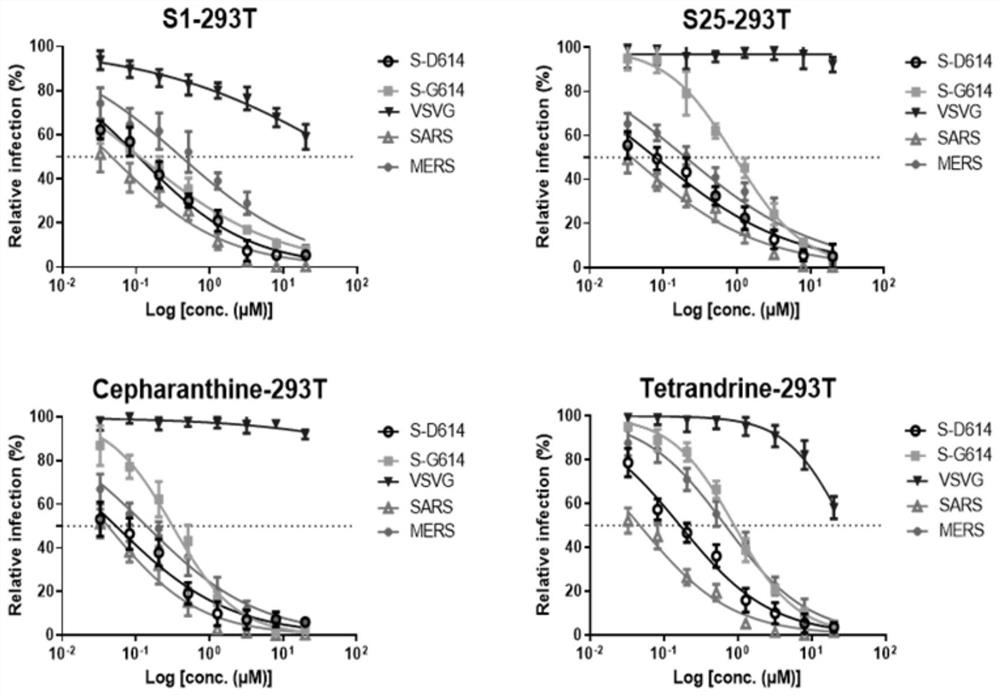
[0068] Embodiment 3: Compound is to the inhibitory activity of different coronavirus infection
[0069] 1. Experimental method
[0070] HEK293T cells were treated with (1×10 4 / well) density was inoculated into 96-well plate, 12h later, the compound to be tested (50μM to 32.7nM) was diluted with a 2.5-fold gradient concentration and incubated with an equal volume of virus supernatant at 37°C for 30min, and then added to the cells. Add the virus control group, the solvent blank control group, stepherine and tetrandrine as positive controls, and each group has 3 replicate wells, and culture at 37°C for 48h. Then add 30 μl 1× cell lysate to each well to lyse the cells, lyse at room temperature for 15 minutes, centrifuge to collect 15 μl supernatant, add 15 μl detection substrate, mix thoroughly, and immediately detect luciferase activity with a microplate reader. According to the inhibition rate of different concentrations of drugs to coronavirus, the half effective concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com