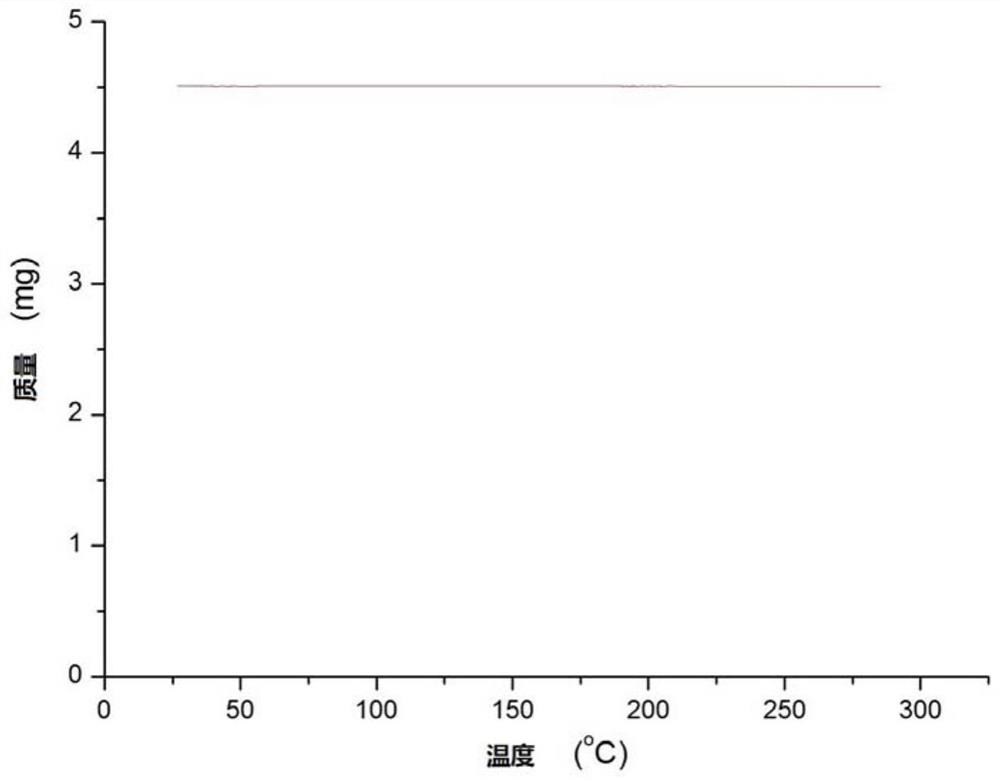
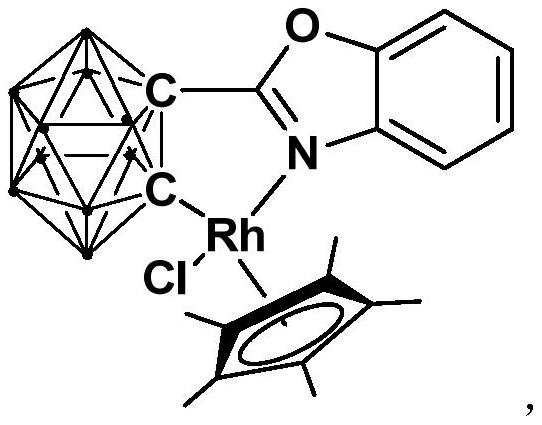
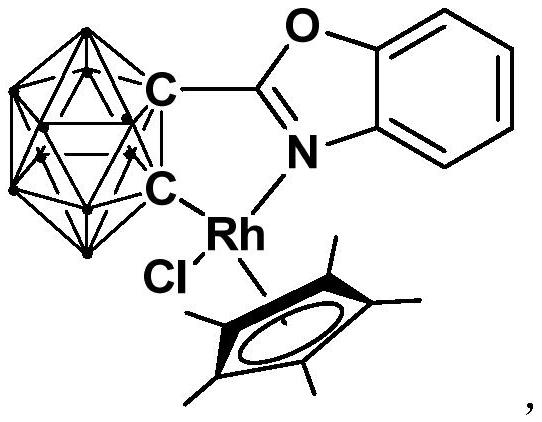
Half-sandwich rhodium complex with ortho-position carborane benzoxazole structure as well as preparation method and application of half-sandwich rhodium complex
A carboryl benzoxazole and rhodium complex technology, which is applied in the preparation of hydroxyl compounds, organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of poor yield and selectivity, Low catalytic activity and other problems, to achieve high yield, high catalytic efficiency and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of above-mentioned semi-sandwich rhodium complex comprises the following steps:
[0031] 1) At -85 to -70°C, add n-BuLi's n-hexane solution dropwise to the ortho carborane o-C 2 B 10 h 12 THF solution, and continue to stir for 20-40min, then the solution is slowly warmed up to room temperature, and then continue to react for 30-60min;
[0032] 2) Add bromobenzoxazole and react at room temperature for 6-8h;
[0033] 3) Add [Cp*RhCl 2 ] 2 , and react at room temperature for 3-5h, and then undergo static filtration, decompression to dry the solvent, and column chromatography to obtain the red target product, a half-sandwich rhodium complex.
[0034] Among them, n-BuLi, o-carborane, bromobenzoxazole and [Cp*RhCl 2 ] 2 The molar ratio is (2.2-3.0):1:1:0.5; during the column chromatography separation process, the eluent used is a mixed reagent composed of petroleum ether and tetrahydrofuran in a volume ratio of (5-10):1.
[0035] The above-menti...
Embodiment 1
[0039] This embodiment is used to synthesize the semi-sandwich rhodium complex [Rh] containing an ortho carboryl benzoxazole structure, and the specific preparation method includes the following steps:
[0040]
[0041] I) Slowly add n-BuLi (1.6M) n-hexane solution (1.00mL, 1.6mmol n-BuLi) dropwise to the ortho carborane o-C at -78°C 2 B 10 h 10 (92.0mg, 0.64mmol) in tetrahydrofuran solution, and kept stirring at this temperature for 30min;
[0042] II) The product solution obtained in step I) was slowly warmed to room temperature, and continued to stir for 1 h, then added bromobenzoxazole (126.7 mg, 0.64 mmol), and reacted at room temperature for 6 h;
[0043] III) the binuclear rhodium compound [Cp*RhCl 2 ] 2 (256.0mg, 0.32mmol) was added into the product solution obtained in step II), and stirred for 3h; / ethyl acetate=6:1), the red target product rhodium (III) complex [Rh] (245.6 mg, yield 72%) was obtained.
[0044] Product characterization results are as follows...
Embodiment 2
[0052] In this embodiment, the rhodium (III) complex [Rh] in Example 1 is used as a catalyst to catalyze the asymmetric reduction reaction of aliphatic ketones, and the specific process is as follows:
[0053]
[0054] Add the ethanol solution of the rhodium (III) complex [Rh] (0.01mmol, 5.3mg) to the butanone solution (10mmol, 0.72g), and pass hydrogen gas under normal pressure as a reducing agent to react, and control the reaction temperature The temperature is 80°C, and the reaction time is 120 minutes; after the reaction is completed, the product mixture is concentrated, separated by silica gel column chromatography, and dried to constant weight to obtain the target product chiral alcohol compound C 4 h 10 O (94% yield), ee>99%.
[0055] Product characterization results are as follows:
[0056] Elemental analysis theoretical value: C 64.82, H 13.60; experimental value: C 64.89, H 13.62, the characterization results show that the chiral alcohol compound C was successfu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com