Method for catalytically synthesizing 1H-imidazole-4-carboxylic acid through inorganic-salt composite catalyst
A technology of composite catalyst and ethyl imidazole formate, which is applied in the field of organic synthesis, can solve the problems of affecting recycling and reusability, increasing industrial production costs, toxicity of Raney nickel, etc., achieves good chemical and physical stability, and reduces process Cost, strong chemical inert effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
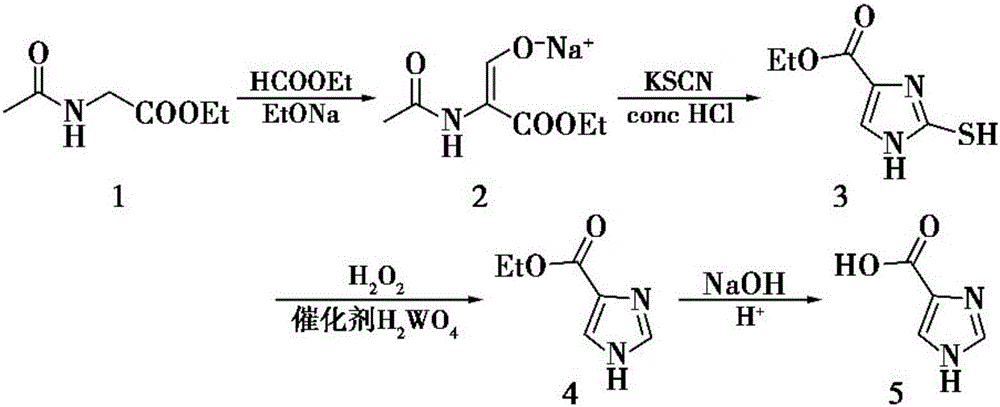
[0041] The first step: the preparation of ethyl 2-mercapto-4-imidazole carboxylate:
[0042] Dissolve ethyl acetylglycinate in the solvent methyl acetate, add sodium ethylate and ethyl formate, wherein the feeding ratio of methyl acetate to ethyl acetylglycinate is 30mL: 5g; the feeding ratio of ethyl acetylglycinate to sodium ethylate is 1 : 5; The feed ratio of ethyl acetylglycinate and ethyl formate is 1g: 30mL;
[0043] After the reaction was completed, potassium thiocyanate was added for dissolving in the gained enol sodium salt, and copper sulfate solution was added, wherein ethyl acetylglycinate: potassium thiocyanate mass ratio was 1:0.5; ethyl acetylglycinate and copper sulfate solution The feed ratio is 1.0g:30mL; after the reaction, the solvent is removed by rotary evaporation to obtain a light yellow powder, which is ethyl 2-mercapto-4-imidazolecarboxylate;
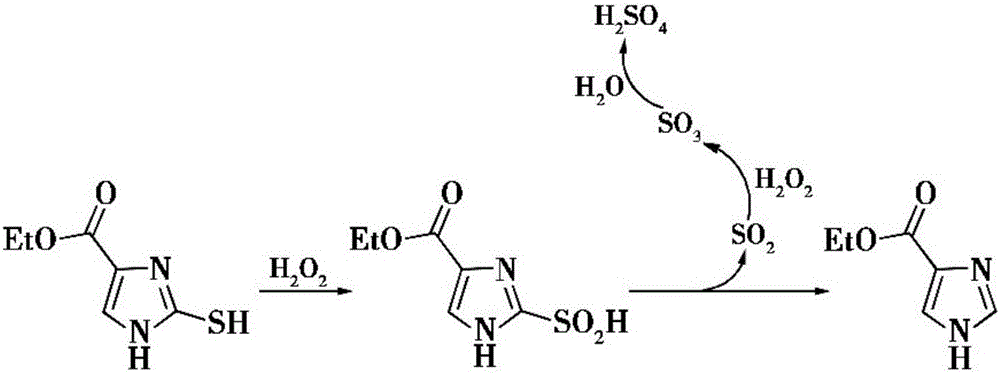
[0044] Second step: the preparation of imidazole-4-formic acid ethyl ester:
[0045] The ethyl 2-mercapto...
Embodiment 2
[0051] The first step: the preparation of ethyl 2-mercapto-4-imidazole carboxylate:
[0052] Dissolve ethyl acetylglycinate in the solvent methyl acetate, add sodium ethylate and ethyl formate, wherein methyl acetate: ethyl acetylglycinate = 30mL: 6g; the ratio of ethyl acetylglycinate to sodium ethylate is 1:8; The feed ratio of ethyl glycine and ethyl formate is 1g:35mL;
[0053] After the reaction was completed, potassium thiocyanate was added to dissolve in the gained enol sodium salt, and copper sulfate solution was added, wherein ethyl acetylglycinate: potassium thiocyanate mass ratio was 1:1; ethyl acetylglycinate: copper sulfate solution= 1.0g: 35mL; After the reaction, the solvent was removed by rotary evaporation to obtain a light yellow powder, which was ethyl 2-mercapto-4-imidazole carboxylate;
[0054] Second step: the preparation of imidazole-4-formic acid ethyl ester:
[0055] The ethyl 2-mercapto-4-imidazoleformate prepared in the first step is dissolved in t...
Embodiment 3
[0061] The first step: the preparation of ethyl 2-mercapto-4-imidazole carboxylate:
[0062] Dissolve ethyl acetylglycinate in solvent methyl acetate, add sodium ethylate and ethyl formate, wherein the feed ratio of methyl acetate and ethyl acetylglycinate is 30mL: 5g; ethyl acetylglycinate and sodium ethylate feed ratio is 1: 8; The feed ratio of ethyl acetylglycinate and ethyl formate is 1g:30mL;
[0063] After the reaction was completed, potassium thiocyanate was added for dissolving in the gained enol sodium salt, and copper sulfate solution was added, wherein ethyl acetylglycinate: potassium thiocyanate mass ratio was 1:1; ethyl acetylglycinate and copper sulfate solution The feed ratio is 1.0g:30mL; after the reaction, the solvent is removed by rotary evaporation to obtain a light yellow powder, which is ethyl 2-mercapto-4-imidazolecarboxylate;
[0064] Second step: the preparation of imidazole-4-formic acid ethyl ester:
[0065] The 2-mercapto-4-imidazole ethyl format...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com