TMPRSS2 mutant protein, expression carrier and expression engineering bacterium thereof, preparation method of expression carrier of TMPRSS2 mutant protein, and preparation method of expression engineering bacterium of TMPRSS2 mutant protein
A protein expression and expression carrier technology, applied in biochemical equipment and methods, carriers, nucleic acid carriers, etc., can solve the problems of high protein difficulty and low protein purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] According to another typical implementation of the embodiments of the present invention, a method for preparing the expression vector of the TMPRSS2 mutant protein is provided, the method comprising:
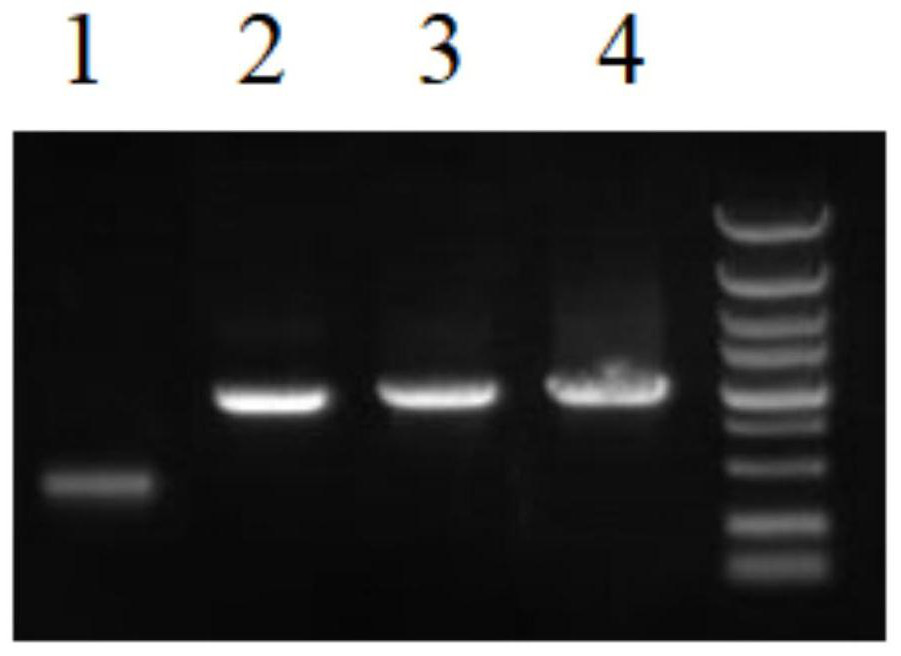
[0061] Obtaining the optimized codon fragment of the TMPRSS2 mutant protein (specifically including: synthesizing the carrier containing the optimized codon fragment of the TMPRSS2 mutant protein, using the pSecTag2A vector as a template, using the primer pair shown in SEQ ID NO.4-5 Perform PCR to obtain an optimized codon fragment of the TMPRSS2 mutant protein), the nucleotide sequence of the optimized codon fragment is shown in SEQ ID NO.3;
[0062] Obtain an expression vector, insert the optimized codon fragment of the TMPRSS2 mutant protein into the expression region of the expression vector, and obtain the TMPRSS2 mutant protein expression vector; specifically include:
[0063] The pSecTag2A vector is double-digested with Hind III / Not I to obtain the restriction vect...
Embodiment 1
[0075] Embodiment 1TMPRSS2 plasmid preparation
[0076] 1. TMPRSS2 codon optimization
[0077] According to NCBI code number (O15393), the nucleic acid sequence of TMPRSS2 is selected as shown in SEQ ID NO.1, and the encoded amino acid is shown in SEQ ID NO.2.
[0078] Considering that different expression systems have different preferences for codons when expressing proteins, the applicant optimized the above codons, and the nucleotide sequence of the optimized gene fragment is shown in SEQ ID NO.3. The amino acid sequence encoded by the nucleotide sequence of the optimized gene fragment is shown in SEQ ID NO.2.
[0079] 2. Obtaining the TMPRSS2 gene fragment
[0080] The optimized gene fragments were synthesized by Wuhan Jinkairui Bioengineering Co., Ltd. Primers were designed according to the optimized nucleic acid sequence of TMPRSS2, and the primers are shown in Table 1.
[0081] Table 1
[0082]
[0083]
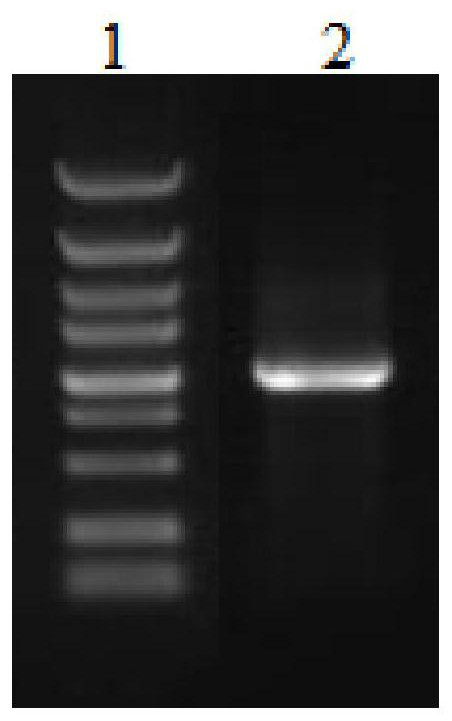
[0084] Using the synthesized optimized gene fragment a...
Embodiment 2
[0106] Example 2 Expression of TMPRSS2 mutant protein in mammalian cells and protein nickel column purification
[0107] 1. Mammalian cell expression
[0108] (1) Transfer the positive monoclonal bacterial liquid to 300ml LB medium to cultivate the large-scale plasmid overnight;
[0109] (2) Prepare two 15ml sterile centrifuge tubes, add 5ml transfection reagent and 100μg sterile plasmid DNA to one of them, and gently blow and mix;
[0110] (3) Standing at room temperature for 10 minutes to prepare the plasmid-carrier complex;
[0111] (4) Take out the 293T cells from the constant temperature shaker, add the prepared plasmid-carrier complex while shaking, and return to 5% CO 2 , Shaking culture in a constant temperature shaker at 37°C.
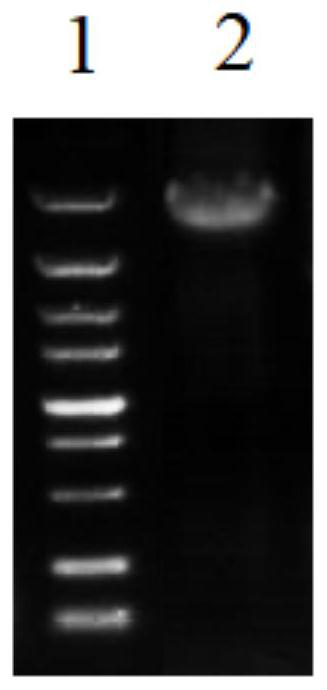
[0112] (5) Shake the flask for 5 to 7 days, harvest the supernatant, and take the secretory supernatant and intracellular supernatant for WB his tag detection to determine whether the protein is expressed. The results are shown in Figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com