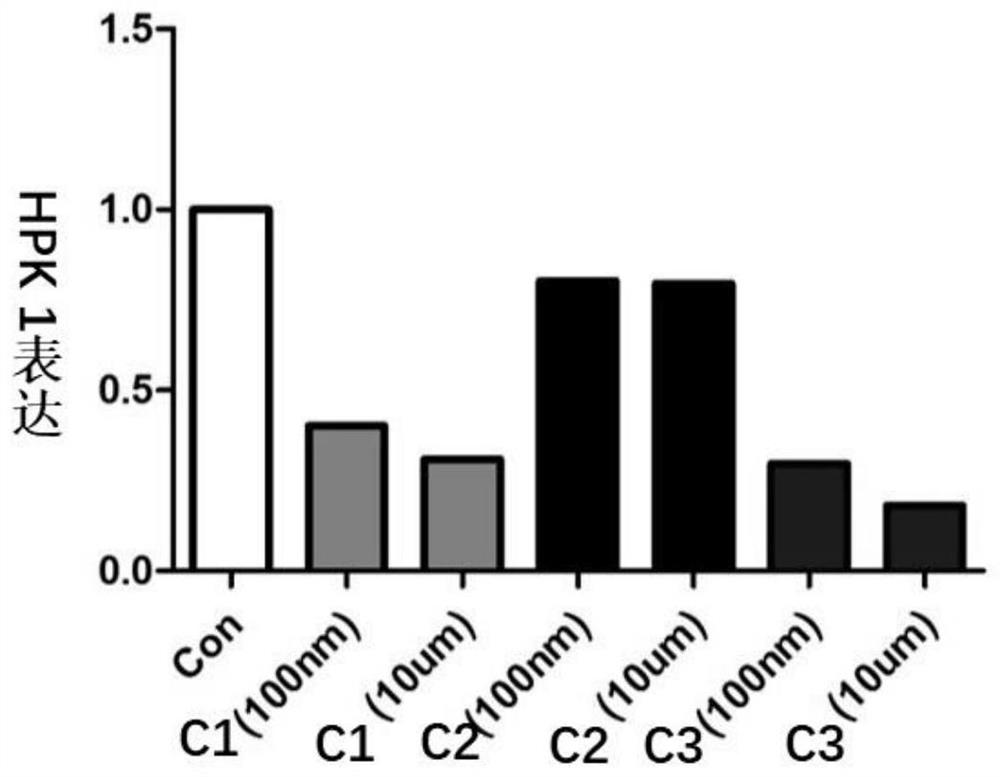
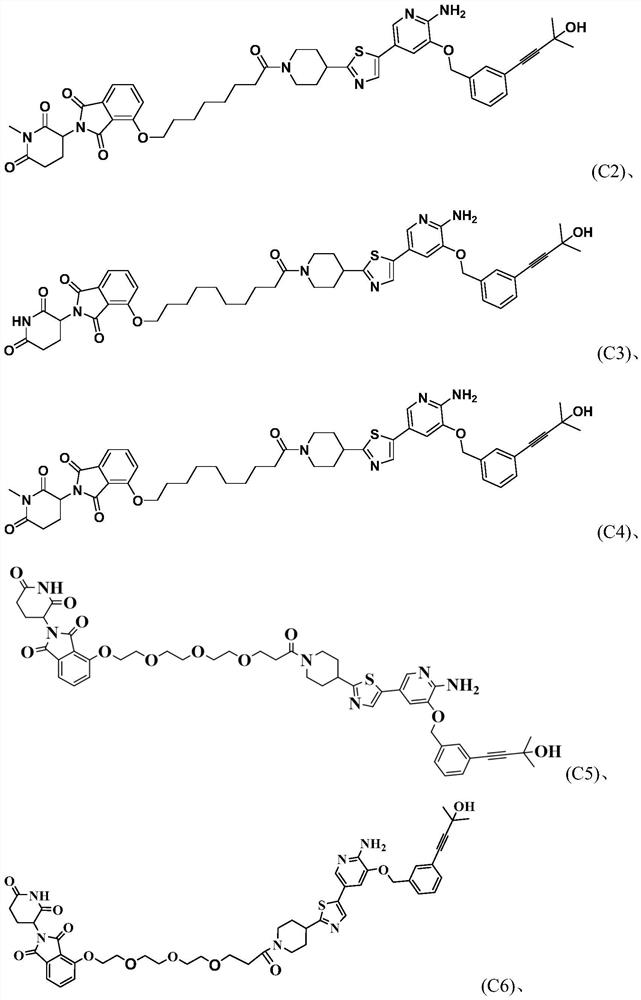
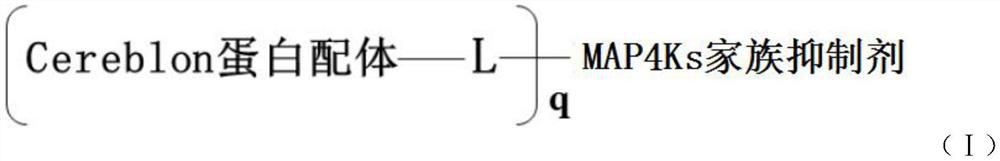PROTAC small molecular compound and application thereof
A technology of compounds and deuterated compounds, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as unclear specific mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0209] The preparation of embodiment 1 compound C1
[0210]
[0211] In a 250ml round bottom flask, add 3-hydroxyphthalic anhydride (2g, 12.2mmol, 1.0equiv) and 3-amino-2,6-piperidinedione hydrochloride (2g, 12.2mmol, 1.0equiv) and An appropriate amount of toluene was added to the reaction system after being thoroughly mixed with triethylamine (1860 ul, 13.4 mmol, 1.1 equiv). After heating to reflux for 12h, stop heating. After the reaction system returned to room temperature, toluene was removed on a rotary evaporator. The crude product was purified by silica gel column chromatography (V methanol: V dichloromethane = 1:40) to obtain the final product 2-(2,6-dioxopiperidin-3-yl)-4-hydroxyiso-1 , 3-diketone 1.9032g, pale yellow solid, yield 57%.
[0212] NMR: 1 H NMR (400MHz, DMSO) δ11.15(s, 1H), 11.07(s, 1H), 7.62(t, J=7.7Hz, 1H), 7.29(d, J=7.1Hz, 1H), 7.22(d ,J=8.4Hz,1H),5.05(dd,J=12.8,5.1Hz,1H),3.04–2.73(m,1H),2.68–2.34(m,1H),2.17–1.73(m,1H). 13 C NMR (100MHz, DMSO)...
Embodiment 2
[0222] The preparation of embodiment 2 compound C2
[0223]
[0224] Dissolve C1 (12mg, 0.014mmol, 1.0equiv) in an appropriate amount of DMF, add potassium carbonate (3.9mg, 0.021mmol, 1.5equiv) and iodomethane (2.4mg, 0.017mmol, 1.2equiv) successively, stir overnight at room temperature, and wait After the reaction is complete, add an appropriate amount of ethyl acetate to dilute, extract with saturated saline for 3 times, take the organic phase and add anhydrous sodium sulfate to dry, filter through a sand core funnel, spin dry, and purify by silica gel column chromatography (V (methanol): V (di Chloromethane)=1:20) to obtain 2-58.2 mg of the final product, with a yield of 68%, LC-MS: m / z=861.
[0225] NMR: 1 H NMR (400MHz, DMSO) δ7.88(s, 1H), 7.84–7.77(m, 1H), 7.76(d, J=1.5Hz, 1H), 7.56–7.48(m, 3H), 7.45–7.36( m,2H),7.36–7.30(m,2H),6.09(s,2H),5.46(s,1H),5.21(s,2H),5.15(dd,J=13.0,5.4Hz,1H),4.42 (d,J=12.9Hz,1H),4.20(t,J=6.3Hz,2H), 3.93(d,J=13.5Hz,1H),3.29–3.09(m,3H),2.9...
Embodiment 3
[0226] The preparation of embodiment 3 compound C3
[0227]
[0228] 2-(2,6-dioxopiperidin-3-yl)-4-hydroxyiso-1,3-dione (300mg, 1.1mmol, 1.0equiv), potassium iodide (18.2mg, 0.1mmol, 0.1equiv ) and sodium bicarbonate (183.8 mg, 2.2 mmol, 2.0 equiv) were dissolved in DMF, and 10-bromo-1-decanol (314 mg, 1.32 mmol, 1.2 equiv) was added to the reaction system. Then heat the reaction system to 80°C, react overnight, stop heating, wait for the reaction system to cool down to room temperature, add an appropriate amount of ethyl acetate to dilute, and extract with saturated saline for 5 times, take the organic phase and add anhydrous sodium sulfate to dry, filter, spin Dry. The crude product was purified by silica gel column chromatography (V (petroleum ether): V (ethyl acetate) = 1: 2) to obtain 253.3 mg of the final product with a yield of 53%, LC-MS: m / z = 431.
[0229]
[0230] Dissolve 3-1 (190mg, 0.32mmol, 1.0equiv), PCC (190mg, 0.64mmol, 2.0equiv) in an appropriate amo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com