Antibody fusion protein, preparation method thereof and application thereof in tumor resistance
A fusion protein and antibody technology, which is applied in the direction of anti-tumor drugs, anti-enzyme immunoglobulin, hybrid immunoglobulin, etc., can solve the problems of slow tumor growth and inability to achieve metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
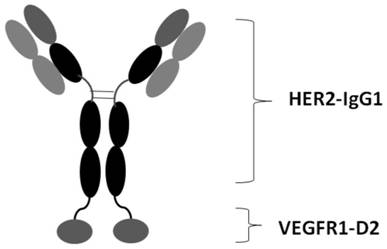
[0093] Example 1. Molecular Construction of Antibody Fusion Protein HD2
[0094] In the present invention, the anti-HER2 monoclonal antibody IgG and the D2 domain of VEGFR1 are connected in series to construct the antibody fusion protein HD2. The D2 domain of VEGFR1 (SEQ ID NO: 14) and the heavy chain (SEQ ID NO: 7) of the anti-HER2 monoclonal antibody are connected through the peptide linker Linker (SEQ ID NO: 9) to obtain the heavy chain of the fusion protein (SEQ ID NO: 9). ID NO: 10). The light chain of the HER2 mAb (SEQ ID NO: 11) remained unchanged. In order to improve the expression efficiency of this molecule in 293E cells, Jinweizhi Company was entrusted to optimize the codons of the nucleic acid sequence of HD2 molecule. Optimization mainly considers factors such as codon preference, GC content, mRNA secondary structure, repeat sequence, etc., and then entrusts Jinweizhi Company to synthesize. After splicing, the HD2 heavy chain nucleic acid sequence is SEQ ID NO:...
Embodiment 2
[0095] Example 2. Expression and purification of antibody fusion protein HD2
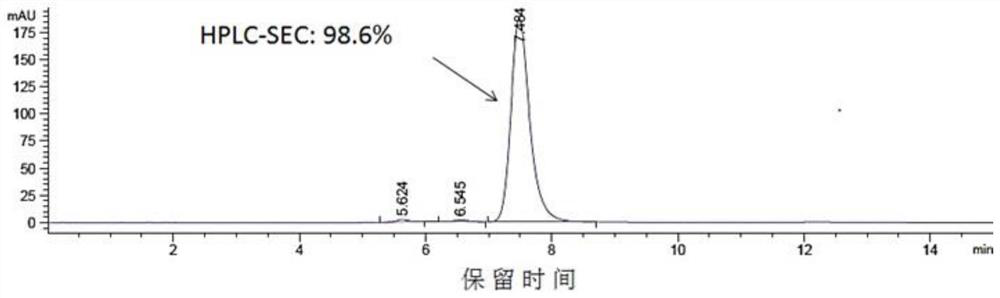
[0096] The heavy chain and light chain DNA fragments of HD2 were respectively cloned into pTT5 vector, and the recombinant plasmids were extracted and co-transfected into CHO cells and / or 293E cells. After the cells have been cultured for 5-7 days, the culture medium is subjected to high-speed centrifugation and vacuum filtration through a microporous membrane, then loaded onto a HiTrap MabSelectSuRe column, and the protein is eluted in one step with an eluent containing 100mM citric acid, pH 3.5, and the target is recovered Samples were dialyzed into PBS pH 7.4. The purified protein was detected by HPLC, and the HPLC-SEC detection patterns of HD2 were as follows: Figure 2A As shown, the molecular state of the antibody is uniform, and the purity of the monomer reaches more than 98%.
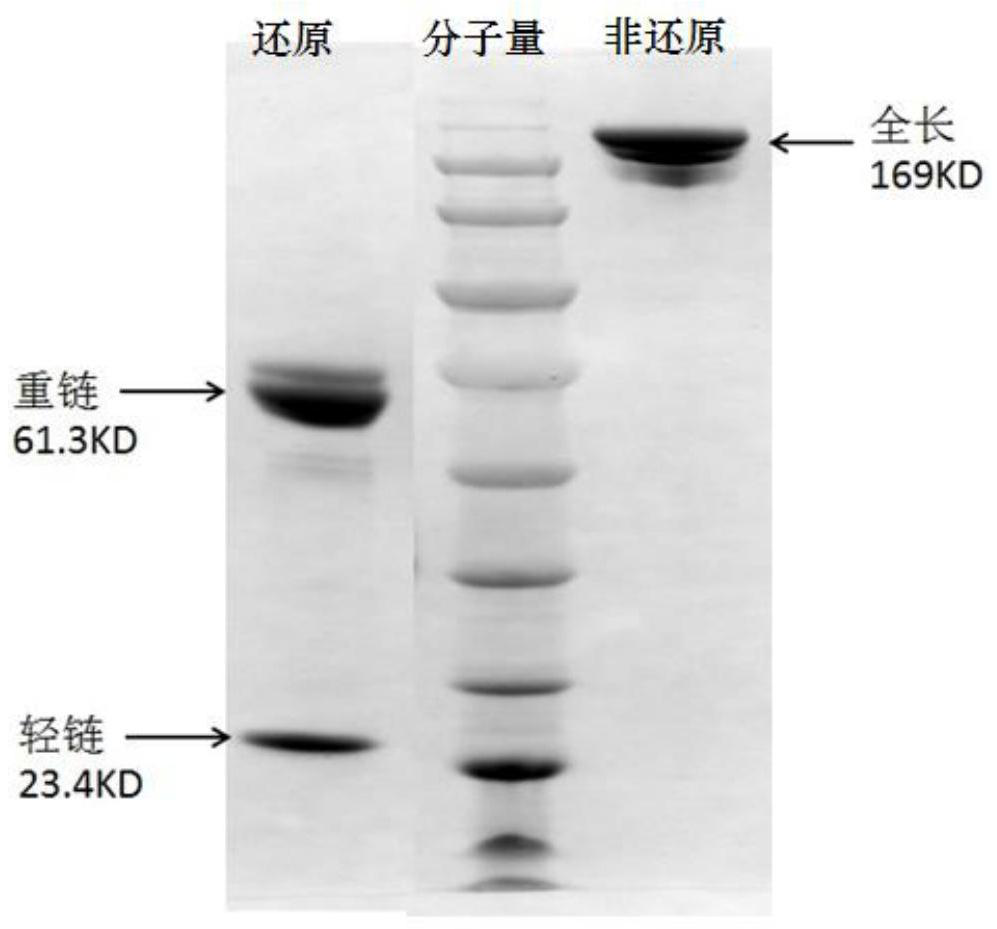
[0097] The purified antibody fusion protein HD2 was added to non-reducing electrophoresis buffer respectively, an...
Embodiment 3
[0098] Example 3. Enzyme-linked immunosorbent assay (ELISA) to measure the affinity of HD2 to HER2 antigen and VEGF
[0099] In order to detect the affinity of HD2 antibody fusion protein and HER2 antigen, the HER2-ECD-His protein produced by Sunshine Guojian was diluted to 250ng / ml with PBS buffer solution of pH 7.4, then 100μl / well was added to the ELISA plate, and incubated at 4°C overnight. The next day, wash the plate twice with PBST, add PBST+1% BSA to each well for blocking, block at 37°C for 1 hour, and wash the plate twice with PBST. Then, the antibody fusion protein HD2 to be detected was diluted with PBS+1% BSA, and the anti-HER2 monoclonal antibody was used as a positive control. The initial concentration was 100 nM, and 12 gradients were gradually diluted 3 times. Incubate at 37°C for 1 hour, wash the plate twice with PBST, add HRP-labeled mouse anti-human Fab antibody, incubate at 37°C for another 40 minutes, wash the plate with PBST three times and pat dry, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com