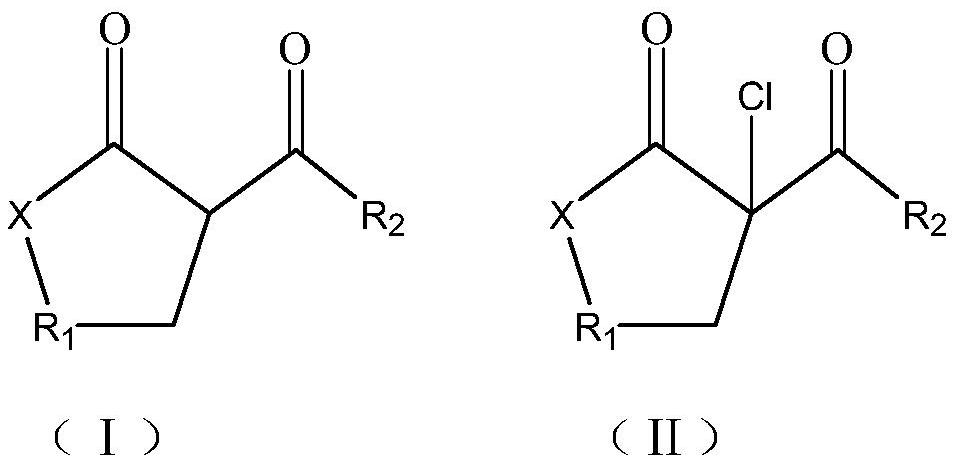
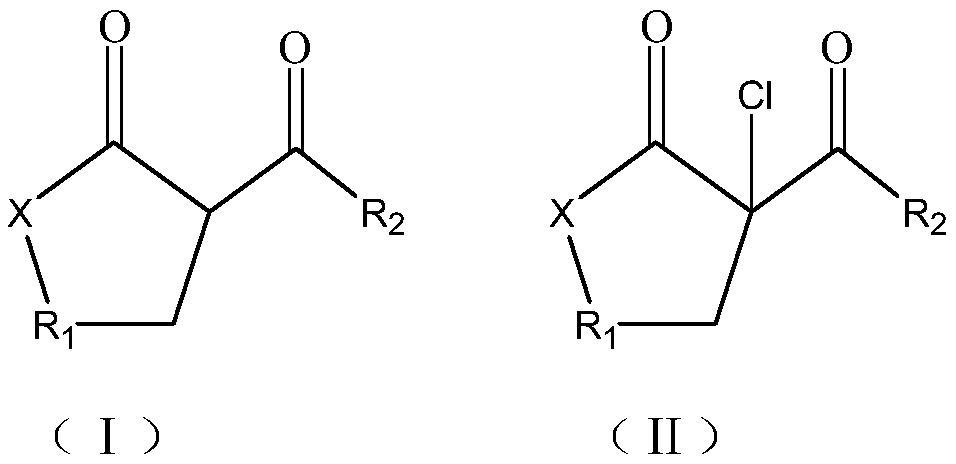
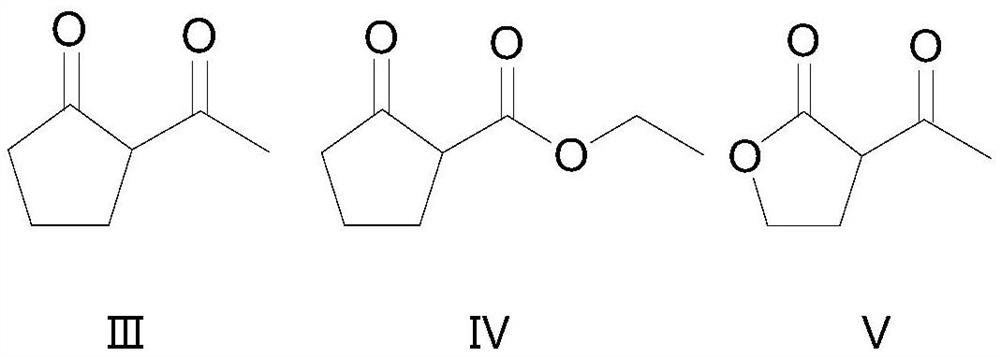
Synthesis method of beta-chloro-alpha, gamma-dicarbonyl compound
A technology of dicarbonyl compounds and synthesis methods, which is applied in the field of synthesis of β-chlorinated α, γ-dicarbonyl compounds, can solve problems such as high reaction cost, low yield, low purity, and environmental pollution, and achieve reduction of side reactions, Simple post-processing, the effect of improving the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of synthetic method of 2-chloro-2-acetyl cyclopentanone:
[0032] Add 200g of 2-acetylcyclopentanone to a 1L four-necked bottle, put in an oil bath at 30°C, add 10.6g of anhydrous aluminum chloride, and feed chlorine gas under stirring conditions at a rate of 17g / h, and set tail gas absorption at the same time. After reacting for 7 hours, gas chromatography detected the completion of the reaction, washed twice with 50 mL of water, separated the liquid, and dried to obtain 254.9 g of crude 2-chloro-2-acetylcyclopentanone. The yield of the target product was 96%, and the gas chromatography purity was 96%.
Embodiment 2
[0034] A kind of synthetic method of 2-chloro-2-ethoxycarbonyl cyclopentanone:
[0035] Add 200g of 2-ethoxycarbonylcyclopentanone to a 1L four-neck flask, put in an oil bath at 30°C, add 8.5g of anhydrous aluminum chloride, and feed chlorine gas under stirring conditions at a feed rate of 11g / h, and set the tail gas absorption at the same time , reacted for 9h, gas chromatography detected the completion of the reaction, washed twice with 50mL of water, separated, and dried to obtain 232.9g of crude product of 2-chloro-2-ethoxycarbonylcyclopentanone. The yield of the target product was 93%, and the gas chromatography purity was 97%.
Embodiment 3
[0037] A kind of synthetic method of 2-chloro-2-acetylbutyrolactone:
[0038] Add 200g of 2-acetylbutyrolactone into a 500L four-necked bottle, put it in an oil bath at 40°C, add 30g of anhydrous aluminum chloride, and feed chlorine gas under stirring conditions at a rate of 30g / h. At the same time, set up tail gas absorption and react After 4 hours, the reaction was detected by gas chromatography, washed twice with 50 mL of water, separated, and dried to obtain 249.5 g of crude 2-chloro-2-acetylbutyrolactone. The yield of the target product was 92%, and the gas chromatography purity was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com