Solid-liquid phase synthesis method of polypeptide drug containing pair of disulfide bonds
A synthesis method and disulfide bond technology, which is applied in the field of solid-liquid phase synthesis of polypeptide drugs, can solve the problems of heavy solvent workload, cumbersome experimental process, troublesome waste disposal, etc., and achieve shortened construction period, simple process operation, and low equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
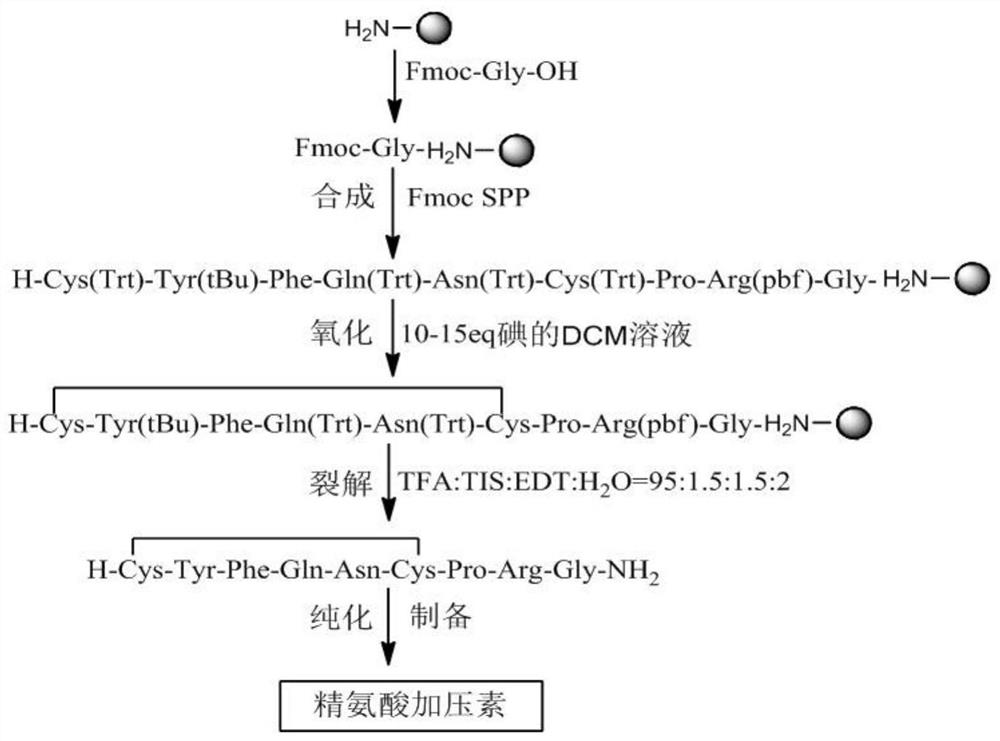
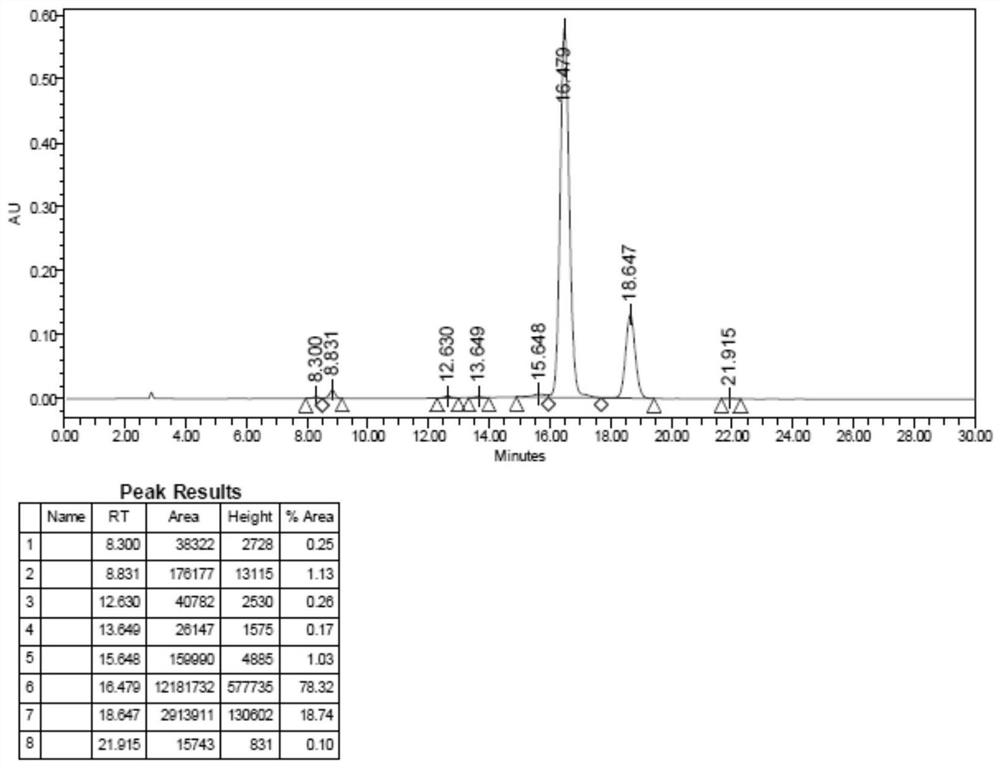
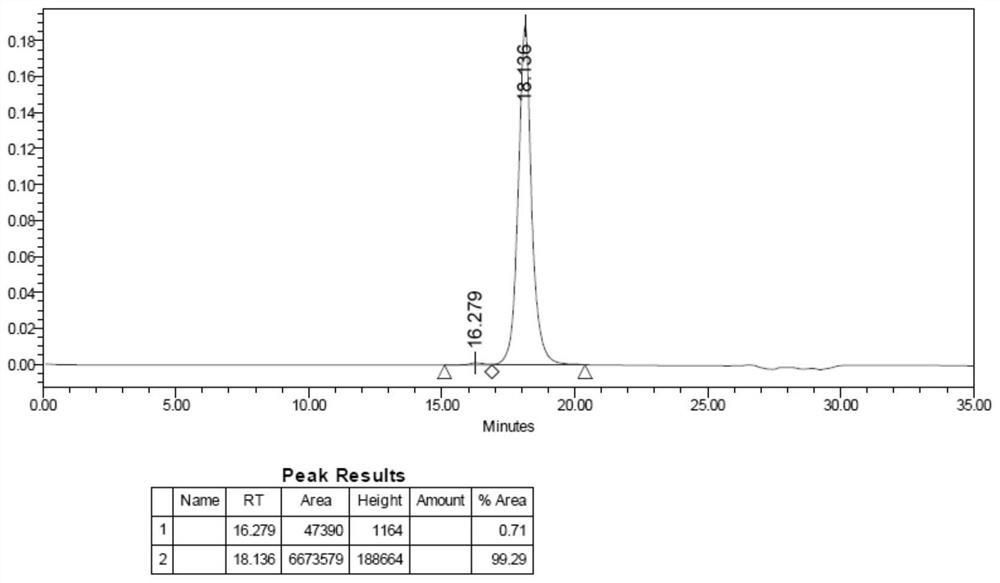
[0055] Example 1 Synthesis of arginine vasopressin
[0056] Flowchart such as Figure 7 shown.
[0057] 1.1 Synthesis
[0058] Weigh 100 g of 0.5 mmol / g Sieber Resin, wash once with DMF, swell with DCM for 30 minutes, add 20% piperidine-DMF by volume, remove Fmoc twice, 5 minutes and 7 minutes respectively. Wash with DMF 6 times, add pre-cooled Fmoc-Gly-OH (297.3, 59.5g, 200mmol) and HOBt (29.7g, 220mmol) DMF solution, stir, then add DIC (126.3, 34.5ml, 220mmol), and react at room temperature 2 hours, ninhydrin detection (resin ball is transparent, it is considered that the reaction is sufficient), after the reaction is completed, filter with suction, wash with DMF 5 times, drain, add 20% piperidine-DMF by volume percentage, remove Fmoc 2 times: 5+7 minute. DMF was washed 6 times, and the DMF solution of Fmoc-Arg(HCl)-OH (432, 86g, 200mmol) and HOBt (29.7g, 220mmol) that had been pre-cooled was added, stirred, and then DIC (126.3, 34.5ml, 220mmol) was added, React at room...
Embodiment 3
[0065] Example 3 Synthesis of desmopressin
[0066] Weigh 100 g of 0.5 mmol / g Sieber Resin resin, wash once with DMF, swell with DCM for 30 minutes, add 20% piperidine-DMF by volume percentage, and remove Fmoc twice for 5 minutes and 7 minutes respectively. Wash with DMF 6 times, add precooled Fmoc-Gly-OH (297.3, 59.5g, 200mmol) and HOBt (29.7g, 220mmol) DMF solution, stir, then add DIC (126.3, 34.5ml, 220mmol), and react at room temperature 2 hours, ninhydrin detection (resin ball is transparent, it is considered that the reaction is sufficient), after the reaction is completed, filter with suction, wash with DMF 5 times, drain, add 20% piperidine-DMF by volume percentage, remove Fmoc 2 times: 5+7 minute. Then sequentially coupled Fmoc-D-Arg(HCl)-OH, Fmoc-Pro-OH, Fmoc-Cys(Mmt)-OH, Fmoc-Asn-OH, Fmoc-Gln-OH, Fmoc-Phe-OH, Fmoc- Tyr-OH, Mpr(Mmt)-OH, finally remove Fmoc, wash with DMF 5 times. DCM was washed 5 times, and methanol shrunk the resin to obtain 177.4 g, with a weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com