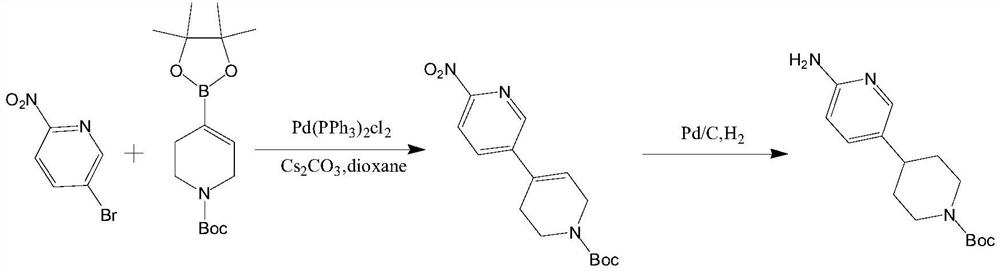
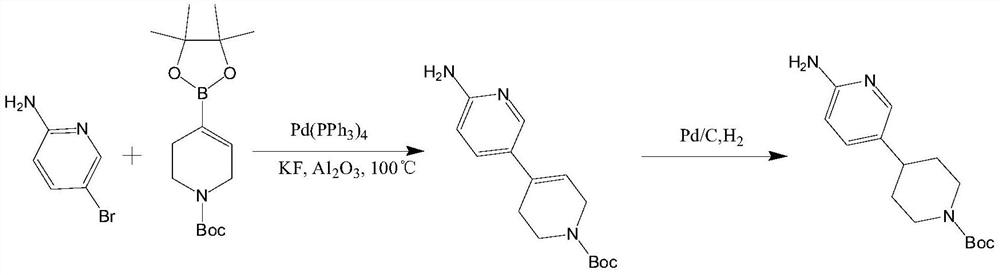
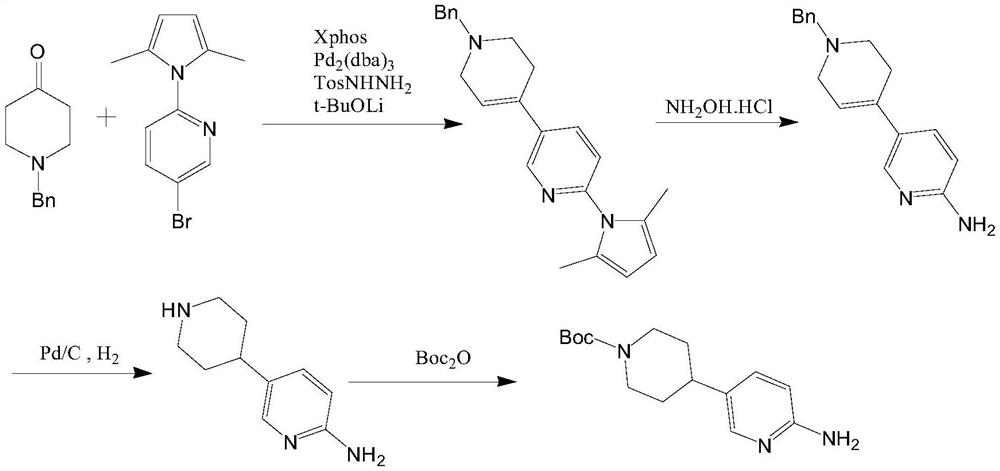
Synthesis method of 4-(6-aminopyridine-3-yl) piperidine-1-tert-butyl formate
A technology of tert-butyl formate and carbamate, applied in the field of synthesis of 4-piperidine-1-tert-butyl carboxylate, can solve the problems of high safety risk and high equipment requirements, and achieve high safety and low equipment requirements , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of 4-(6-fluorenylamino-3-pyridyl)-4-hydroxyl-N-tert-butoxycarbonylpiperidine
[0040] Under nitrogen protection, add 39.5g of fluorenylmethyl N-(5-bromopyridin-2-yl)carbamate into 400ml of anhydrous THF, drop 100ml of isopropylmagnesium chloride and lithium chloride at 0-5°C solution (1.3M), dropwise and keep warm for 2 hours to obtain the Grignard reagent.
[0041] Under the protection of nitrogen, 17.9g of N-tert-butoxycarbonyl-4-piperidone was dissolved in 100ml of anhydrous THF, the above-mentioned Grignard reagent was added dropwise, the temperature was controlled at 10-15°C, and the reaction was kept for 3h. After the TLC reaction is completed, add 500ml of saturated ammonium chloride solution, extract with 300ml of ethyl acetate, dry and evaporate to dryness, add 150ml of methyl tert-butyl ether to reflux for beating for 1h, drop to -5~0°C, filter with suction, and dry 41.9 g of the product was obtained with a yield of 90.3% and a purity of 98.6%. ...
Embodiment 2
[0045] (1) Synthesis of 4-(6-fluorenylamino-3-pyridyl)-4-hydroxyl-N-tert-butoxycarbonylpiperidine
[0046] Under nitrogen protection, add 59.3g of fluorenylmethyl N-(5-bromopyridin-2-yl)carbamate into 600ml of anhydrous 2-methyltetrahydrofuran, drop 145ml of isopropyl Magnesium chloride lithium chloride solution (1.3M), after dripping, keep warm for 2 hours to obtain the Grignard reagent.
[0047] Under the protection of nitrogen, dissolve 29.8g of N-tert-butoxycarbonyl-4-piperidone in 150ml of anhydrous 2-methyltetrahydrofuran, add the above-mentioned Grignard reagent dropwise, control the temperature at 5-10°C, keep the temperature for 3h, and perform TLC reaction After completion, add 750ml of saturated ammonium chloride solution, extract with 450ml of ethyl acetate, dry and evaporate to dryness, add 200ml of methyl tert-butyl ether to reflux for beating for 1 hour, drop to -5~0°C, filter with suction, and dry to obtain the product 70.3g, yield 91.1%, purity 98.7%.
[0048]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com