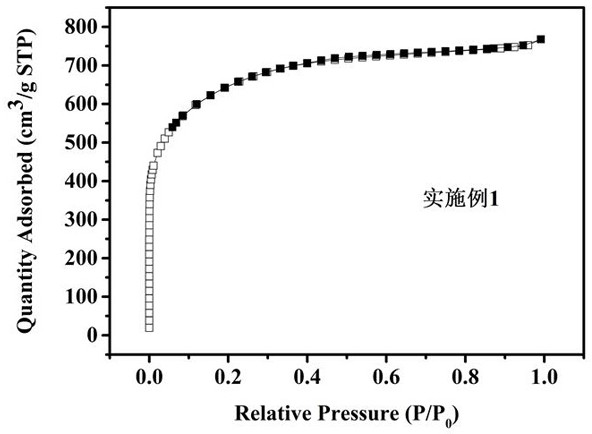
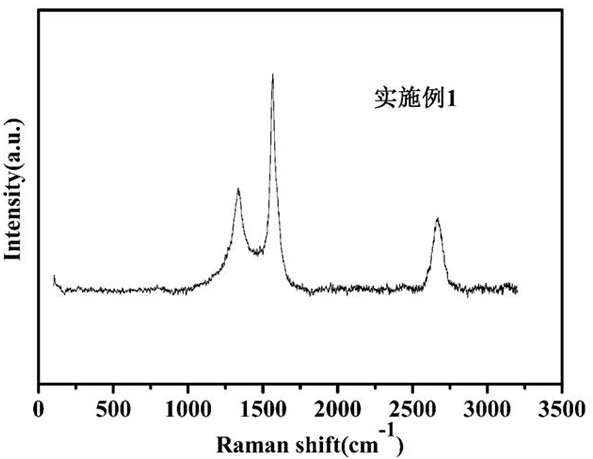
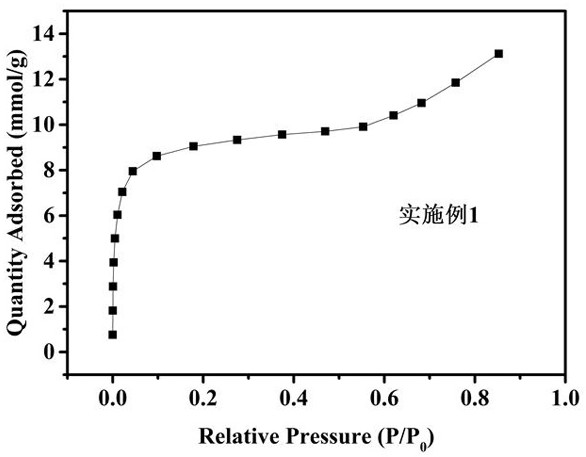
Preparation and application of a glucose-based porous carbon material
A technology of glucose and porous carbon, applied in other chemical processes, separation methods, separation of dispersed particles, etc., can solve the problems of low environmental pollution and high yield, and achieve the effects of high adsorption capacity, high specific surface area, and good graphitization degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Dissolve 4 g of glucose in 15 mL of deionized water, stir at room temperature for 15 min to obtain solution A; dissolve 0.09 g of iron powder and 0.23 g of trimesic acid in 27 mL of deionized water, and add 0.14 mL of hydrogen successively Hydrofluoric acid (HF, 40 wt%) and 0.07 mL nitric acid (HNO 3 , 65 wt%), stirred at room temperature for 15 min to obtain solution B; added solution A to solution B, and stirred at room temperature for 20 min; transferred the resulting mixed solution into a stainless steel autoclave, heated to 160 °C for 10 h; centrifuged and washed the product , soaked twice in ethanol at 60 °C for 10 h each time, and then dried in vacuum at 150 °C for 10 h.
[0034] (2) Add KOH (sample / KOH=1:3) to the product obtained above, mix well, put it into a tube furnace, raise the temperature to 800 °C at 5 °C / min under nitrogen protection, and maintain it for 1 h, then cool down ; then washed with 2 mol / L hydrochloric acid, washed with water, and final...
Embodiment 2
[0036] (1) Dissolve 4 g of glucose in 15 mL of deionized water, stir at room temperature for 15 min to obtain solution A; dissolve 0.20 g of iron powder and 0.50 g of trimesic acid in 27 mL of deionized water, and add 0.32 mL of hydrogen successively Hydrofluoric acid (HF, 40 wt%) and 0.15 mL nitric acid (HNO 3 , 66 wt%), stirred at room temperature for 15 min to obtain solution B; added solution A to solution B, and stirred at room temperature for 20 min; transferred the resulting mixed solution into a stainless steel autoclave, heated to 160 °C for 10 h; centrifuged and washed the product , soaked twice in ethanol at 60 °C for 10 h each time, and then dried in vacuum at 150 °C for 10 h.
[0037] (2) Add KOH (sample / KOH=1:3) to the product obtained above, mix well, put it into a tube furnace, raise the temperature to 700 °C at 5 °C / min under nitrogen protection, and maintain it for 1 h, then cool down ; then washed with 2 mol / L hydrochloric acid, washed with water, and final...
Embodiment 3
[0039] (1) Dissolve 4 g of glucose in 15 mL of deionized water, stir at room temperature for 10 min to obtain solution A; dissolve 0.20 g of iron powder and 0.50 g of trimesic acid in 27 mL of deionized water, and add 0.32 mL of hydrogen successively Hydrofluoric acid (HF, 40 wt%) and 0.15 mL nitric acid (HNO 3 , 67 wt%), stirred at room temperature for 10 min to obtain solution B; added solution A to solution B, and stirred at room temperature for 15 min; transferred the resulting mixed solution into a stainless steel autoclave, heated to 170 °C for 10 h; centrifuged and washed the product , soaked twice in ethanol at 60 °C for 8 h each time, and then dried in vacuum at 150 °C for 10 h.
[0040] (2) Add KOH (sample / KOH=1:4) to the product obtained above, mix well and put it into a tube furnace, raise the temperature to 800 ℃ at 5 ℃ / min under the protection of nitrogen and maintain it for 1 h, then cool down ; then washed with 2 mol / L hydrochloric acid, washed with water, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com