Neuroprotective drug for cerebral arterial thrombosis
A technology for ischemic stroke and neuroprotection, applied in drug combinations, neurological diseases, pharmaceutical formulations, etc., to reduce or stabilize the disease, reduce ischemic infarct size, and improve behavior.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Example 1 tFNAs
[0033] 1. Synthesis method
[0034] Dissolve the four DNA single strands (S1, S2, S3, S4) in TM Buffer (10mM Tris-HCl, 50mM MgCl 2 , pH=8.0), so that the final concentration of the four DNA single strands is 1000nM, mix well, heat quickly to 95°C and keep it for 10 minutes, then quickly cool down to 4°C and keep it for more than 20 minutes, you can get tetrahedral skeleton nucleic acid TFNAS.
[0035] The sequences of the four single strands (5'→3') are as follows:
[0036] S1:
[0037]
[0038] S2:
[0039]
[0040] S3:
[0041]
[0042] S4:
[0043]
[0044] 2. Identification
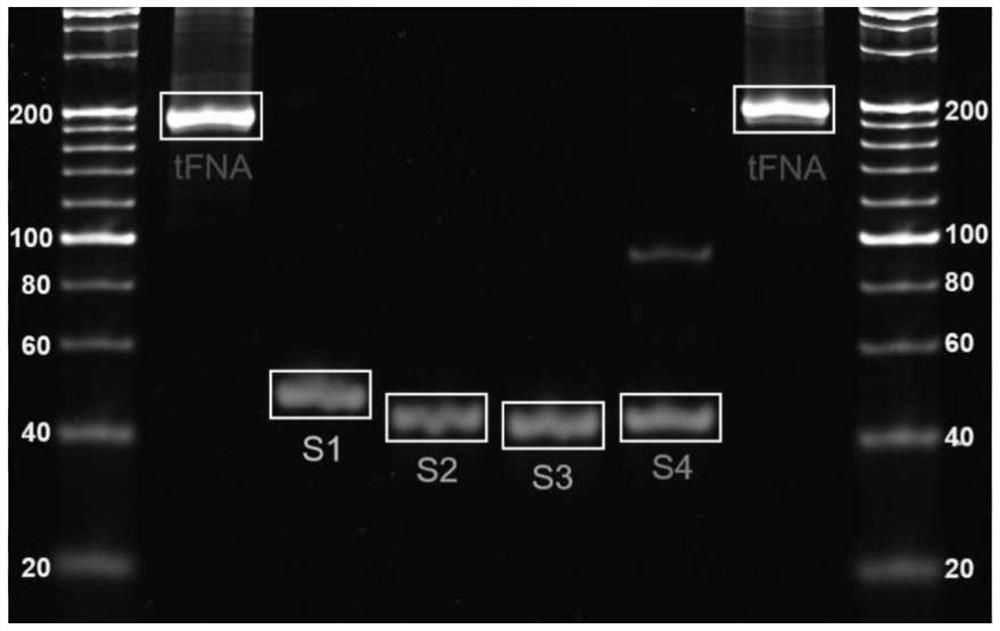
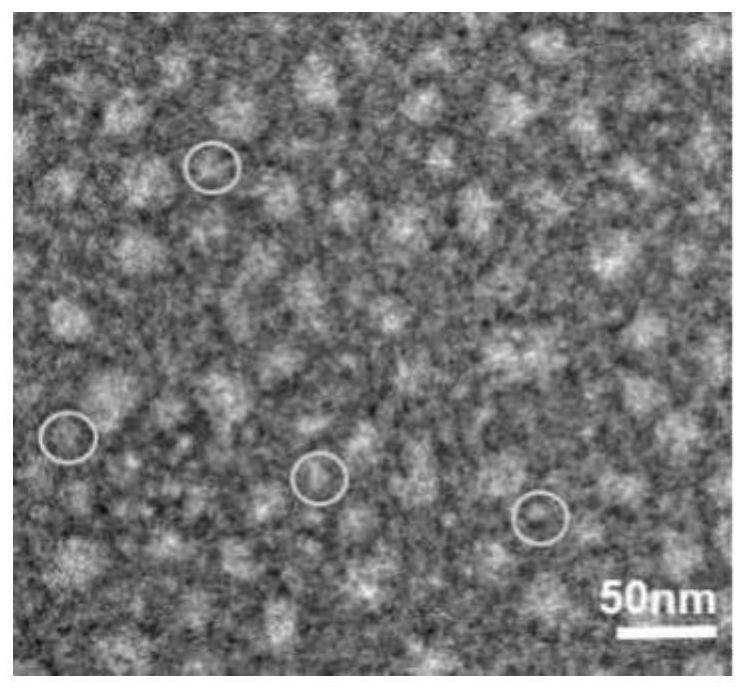
[0045] From the results of PAGE gel, it can be seen that the size of TFNAS is about 200bp ( figure 1 ); Scattered point-like objects can be seen by transmission electron microscopy, and some point-like objects can be observed to present a tetrahedral shape ( figure 2 ).
[0046] From the aforementioned identification results, it can be consi...
experiment example 1
[0048] Experimental example 1 Neuroprotective effect of tFNAs
[0049] 1. Method
[0050] (1) Animal treatment in groups
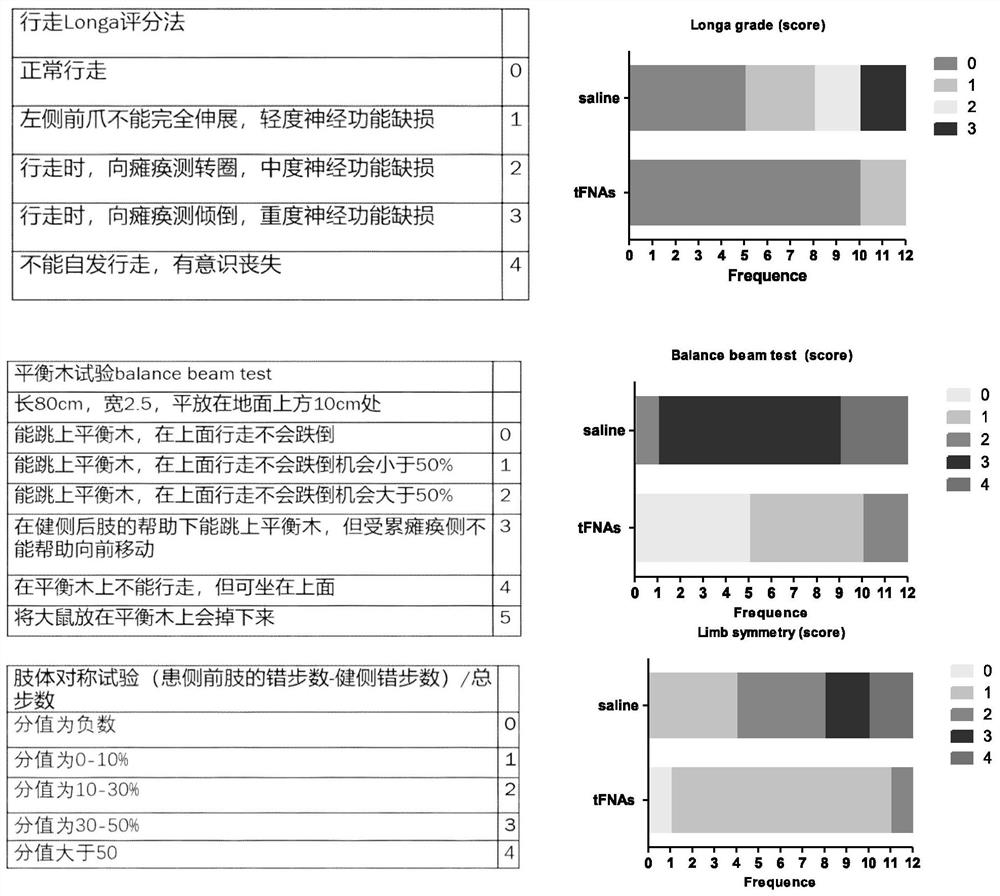
[0051] SD male rats weighing 210-230 g were divided into 3 groups.
[0052] a. Sham operation group (sham): the right common carotid artery of rats was permanently ligated.
[0053] b. Normal saline group (saline, SA): Permanent ligation of the right common carotid artery and external carotid artery of rats, using a 0.4mm diameter silicone head thread plug to insert the internal carotid artery from the bifurcation of the common carotid artery, and plug it into the back of the cranium Middle cerebral artery for 1 hour. When the middle cerebral artery was blocked, 200 microliters of normal saline was injected into the tail vein at the same time. After 1 hour, the thread plug was removed.
[0054] c. Experimental group (tFNAs): The right common carotid artery and external carotid artery of rats were ligated for a long time, and a 0.4mm diameter silicone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com