Preparation method of (R)-3-cyclohexeneformic acid
A technology of cyclohexene carboxylic acid and dimethyl ring, which is applied in the field of preparation of -3-cyclohexene carboxylic acid, can solve the problems of expensive raw materials, cumbersome reaction process, and difficult filtration of salt, and achieve high atom utilization rate and reaction Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of (R)-3-cyclohexenecarboxylic acid
[0021]
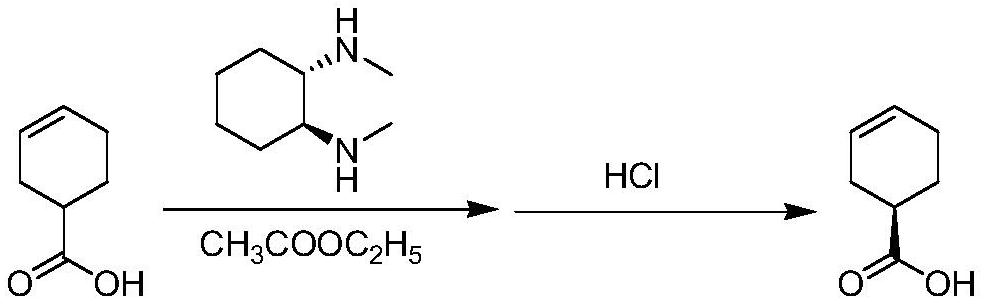
[0022] Into a 2000mL reaction flask, add 200.0g 3-cyclohexenecarboxylic acid (1.585mol) and 1000mL ethyl acetate, control the temperature to 45-50℃, and add 112.8g (1S, 2S)-N,N′-di A solution formed by dissolving methylcyclohexanediamine (0.793mol, 0.5eq) in 200mL ethyl acetate took 2.5 hours. After the dropwise addition, the temperature was raised to reflux for 4.0 hours, and the temperature was gradually lowered to 10-15°C. Filtration gave white crystalline solid (R)-3-cyclohexenecarboxylic acid-(1S,2S)-N,N'-dimethylcyclohexanediamine salt. Put the filter cake back into the reaction flask, add ethyl acetate and 6M hydrochloric acid aqueous solution, adjust the pH=1-2, stir for 0.5 hours, and separate layers when the pH value does not change. The organic layer was dried over anhydrous sodium sulfate, concentrated by filtration, added 40g of sulfolane, and distilled under reduced pressure (-0.099Mpa, 79~85°C)...
Embodiment 2
[0024]
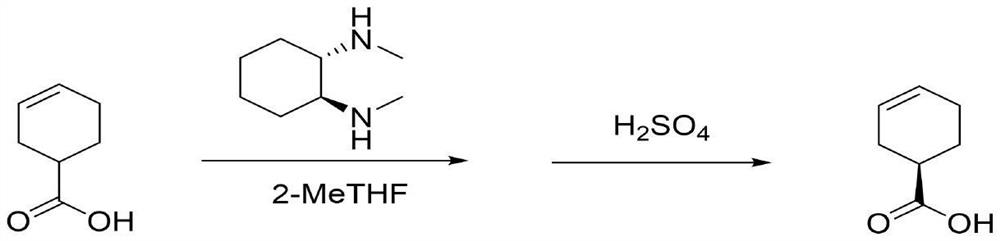
[0025] Into a 2000mL reaction flask, add 200.0g 3-cyclohexenecarboxylic acid (1.585mol) and 800mL 2-methyltetrahydrofuran and mix, control the temperature to 45~50℃, add 112.8g (0.793mol, 0.5eq) (1S ,2S)-N,N′-Dimethylcyclohexanediamine was dissolved in 200mL 2-methyltetrahydrofuran to form a solution. After the dropwise addition, the temperature was raised to reflux for 4 hours, and the temperature was gradually lowered to 5-10°C. The white crystalline solid (R)-3-cyclohexenecarboxylic acid-(1S,2S)-N,N'-dimethylcyclohexanediamine salt was obtained by filtration. Put the filter cake back into the reaction flask, add 500mL 2-methyltetrahydrofuran, control the temperature not to exceed 20°C, slowly add 20% sulfuric acid aqueous solution dropwise, adjust the pH=2-3, stir for 0.5 hours, and separate when the pH value does not change . The organic layer was dried over anhydrous sodium sulfate, concentrated by filtration, added 40g of sulfolane, and distilled under reduc...
Embodiment 3
[0027]
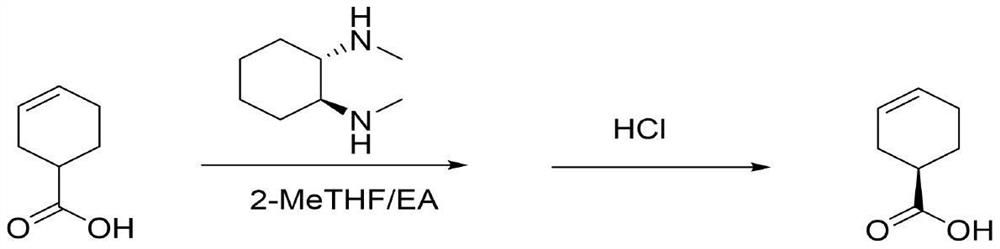
[0028] Add 200.0g of 3-cyclohexenecarboxylic acid (1.585mol) and 800mL of 2-methyltetrahydrofuran into a 2000mL reaction flask, mix them, control the temperature to 45-50°C, and add 112.8g (0.793mol, 0.5eq) (1S ,2S)-N,N′-Dimethylcyclohexanediamine was dissolved in 200mL of ethyl acetate to form a solution. After the dropwise addition, the temperature was raised to reflux for 4 hours, and the temperature was gradually lowered to 5-10°C. The white crystalline solid (R)-3-cyclohexenecarboxylic acid-(1S,2S)-N,N'-dimethylcyclohexanediamine salt was obtained by filtration. Put the filter cake back into the reaction flask, add 500mL ethyl acetate, control the temperature not to exceed 20°C, slowly add 6M hydrochloric acid aqueous solution dropwise, adjust the pH=1-2, stir for 0.5 hours, and separate when the pH value does not change. The organic layer was dried over anhydrous sodium sulfate, concentrated by filtration, added 40g of sulfolane, and distilled under reduced p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com