Preparation method of 7-amino-6-demethylation-6-deoxy tetracycline and minocycline hydrochloride
A technology of minocycline hydrochloride and deoxytetracycline, which is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, organic chemical methods and other directions, can solve the problems of small safety risk and complicated process operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0172] The present invention provides a kind of preparation method of 7-amino-6-desmethyl-6-deoxytetracycline, described method comprises steps:
[0173]
[0174] (1) 6-demethyl-6-deoxytetracycline reacts with a chlorinated reagent to obtain a chlorinated product; the chlorinated product reacts with an azo reagent to obtain a chlorinated product containing 7-p-benzenesulfonate Acid azo-11a-chloro-6-demethyl-6-deoxytetracycline reaction solution;
[0175] (2) adding a reducing agent to the reaction solution containing 7-p-benzenesulfonic acid azo-11a-chloro-6-demethyl-6-deoxytetracycline obtained in step (1), and reacting to obtain 7 -Amino-6-desmethyl-6-deoxytetracycline.
[0176] In the preparation method of 7-amino-6-demethyl-6-deoxytetracycline of the present invention, 6-demethyl-6-deoxytetracycline also includes the form of its salt, preferably, the 6 - demethyl-6-deoxytetracycline includes 6-desmethyl-6-deoxytetracycline sulfate.
[0177] In a preferred example of ...
Embodiment 1
[0324] Example 1: Synthesis of 7-amino-6-demethyl-6-deoxytetracycline
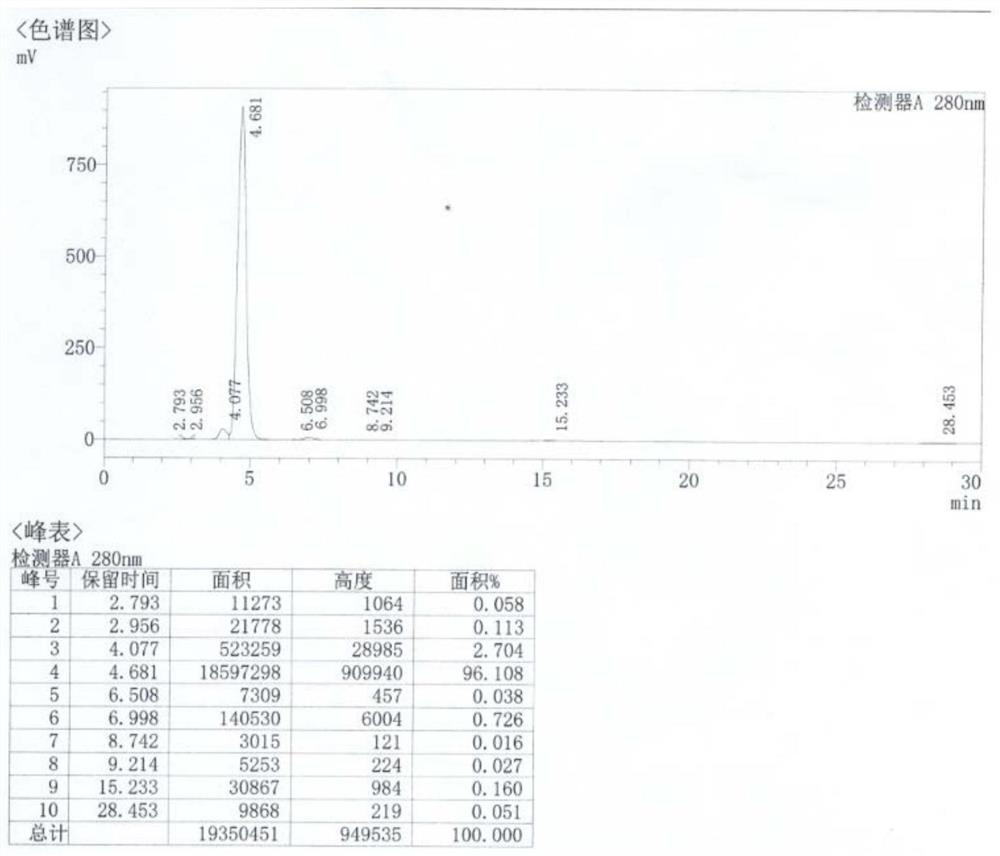
[0325] Add 30g of 6-desmethyl-6-deoxytetracycline sulfate and 9.38g of N-chlorosuccinimide to 330ml of water, stir and react at 20-30°C for 1h, cool down to 0-5°C, and pour into the reaction system Add 31g diazonium salt of p-aminobenzenesulfonate, through NaHCO 3 Adjust the pH to 6.6-7.4 to obtain a reaction solution containing the product 7-azo-p-benzenesulfonate-11a-chloro-6-demethyl-6-deoxytetracycline, and then add hydrochloric acid dropwise to adjust the pH of the reaction solution=2.0~ 3.0, add 39.6g of stannous chloride, and react at 20-30°C for 3 hours to obtain a reaction solution, adjust the pH of the reaction solution to 6.8-7.2 with sodium hydroxide, use membrane separation equipment, and select a filter with a molecular weight of figure 1 shown.
Embodiment 2
[0326] Embodiment 2: the synthesis of minocycline hydrochloride
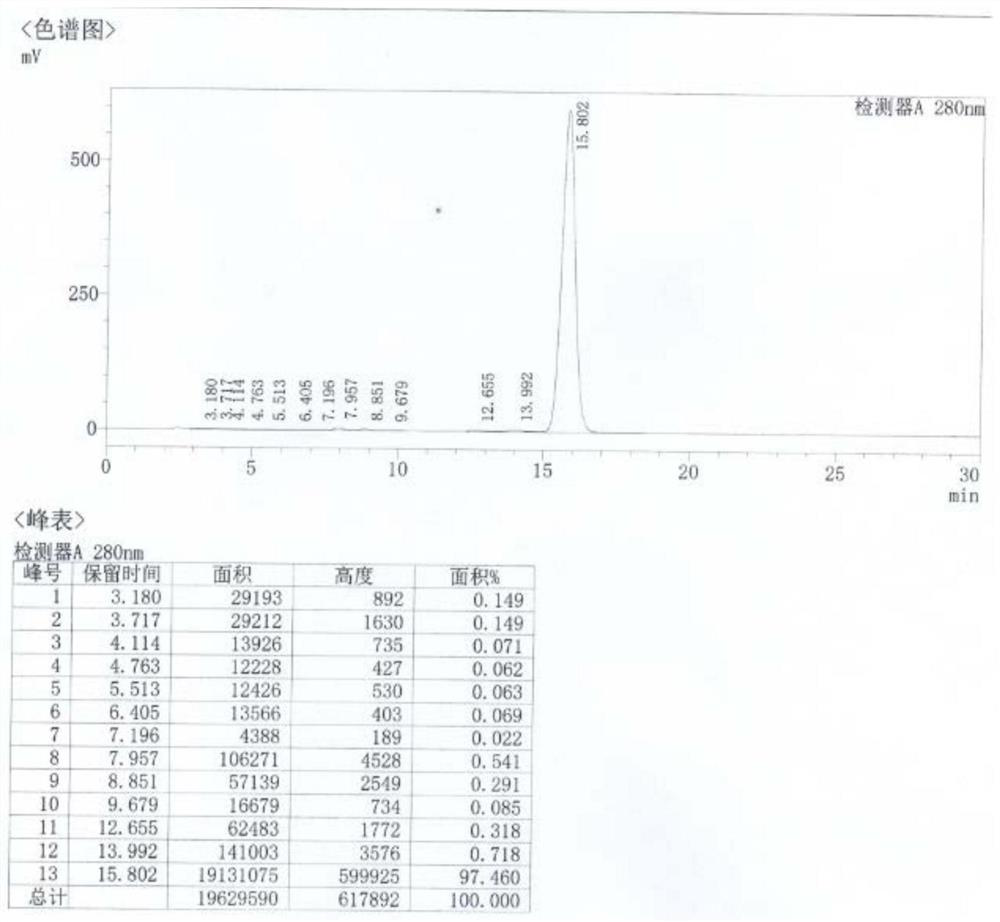
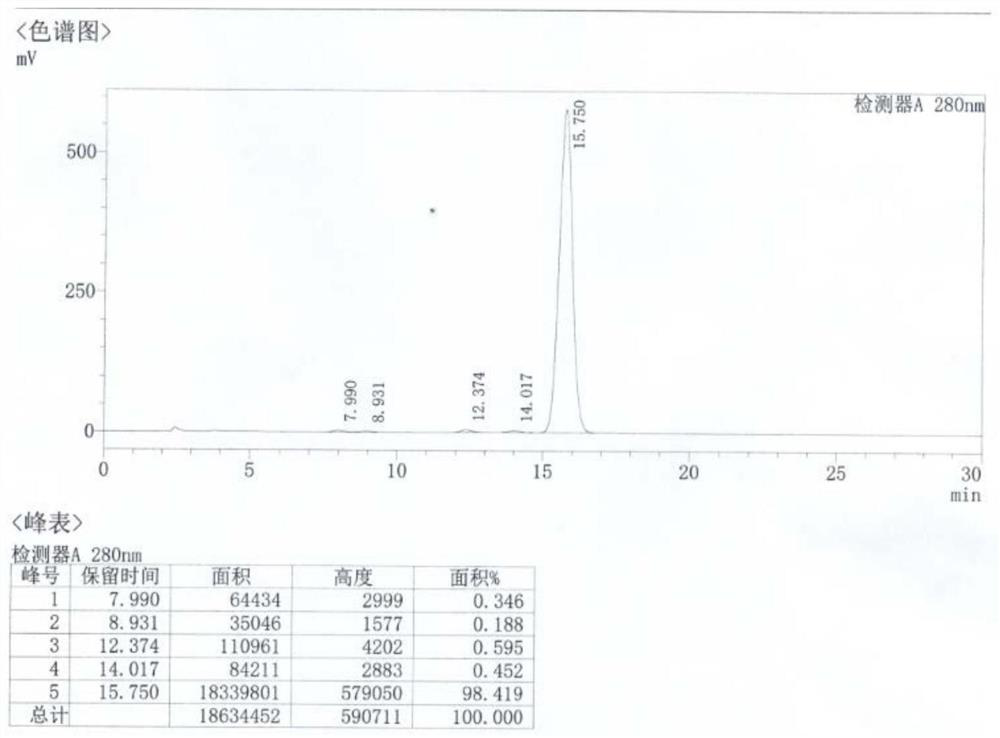
[0327] (1) In a clean four-necked bottle, add 80mL of ethylene glycol monomethyl ether and 20g of 7-amino-6-desmethyl-6-deoxytetracycline prepared in Example 1, add 13.8g of concentrated hydrochloric acid dropwise, and stir thoroughly Dissolve, transfer all the solution to a hydrogenation tank, add 12g of 5wt% Pd / C (50%), control the temperature at 20-25°C, and pass hydrogen (the hydrogen pressure is 7.0-9.0kg / cm 2 reaction liquid level), after adding 42.0 g of formaldehyde aqueous solution (the weight fraction of formaldehyde is 40%), heat preservation and pressure reaction for 3 hours, the reaction is completed, and the reaction liquid is obtained.
[0328] (2) In a clean four-neck bottle, add 1L of clean water, cool down to 0-10°C, add the reaction solution obtained in step (1), add ammonia water dropwise to adjust pH=3.8-4.0, crystallize at 0-10°C for 2 hours, pump Filter, obtain minocycline hydrochloride c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com