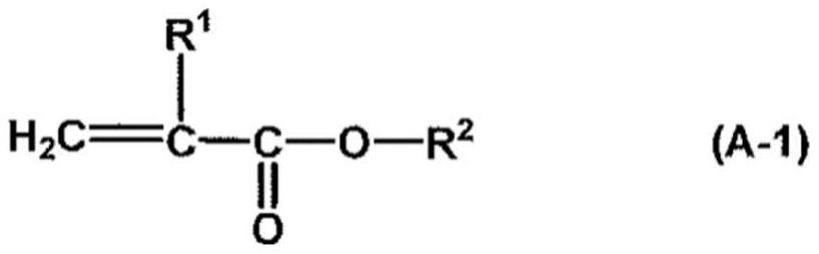
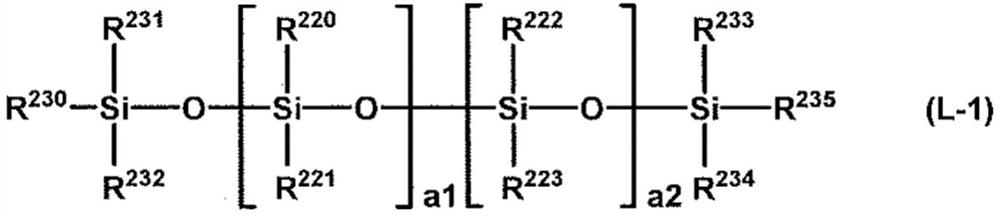
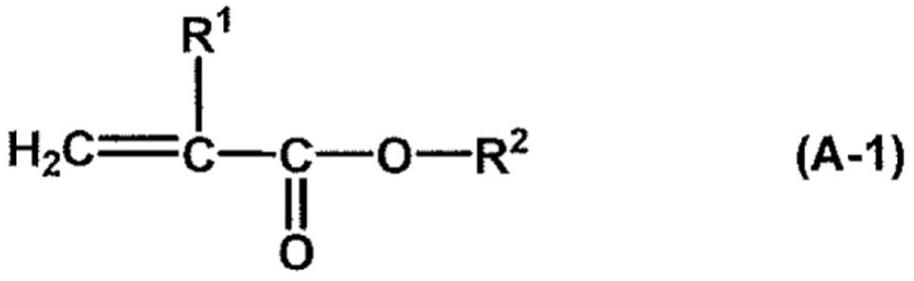
Water-repellent agent composition for fibers, water-repellent textile product, and production method for water-repellent textile product
A technology of waterproofing agent and composition, which is applied in the direction of repelling fiber, fiber treatment, textile and papermaking to liquid, etc., to achieve the effect of durable waterproofness, sufficient waterproofness and excellent initial waterproofness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0355] Hereinafter, the present invention is further illustrated by examples, but the present invention is not limited by these examples.
[0356]
[0357] Using the materials shown in Table 1 and Table 2 {in the table, the numerical value indicates (g)}, polymerization was performed in the procedure shown below to obtain an acrylic resin dispersion.
Synthetic example 1
[0359] 15 g of stearyl acrylate, 5 g of stearyl methacrylate, 0.5 g of Surfynol 465 (manufactured by Nissin Chemical Co., Ltd., HLB=13), and Surfynol 440 (manufactured by Nissin Chemical Co., Ltd., HLB=8) were added to a 0.5 L autoclave. 1 g, Noigen XL-40 (manufactured by Daiichi Kogyo Pharmaceutical Co., Ltd., polyoxyalkylene branched decyl ether, HLB=10.5) 0.5 g, 1 g of stearyl trimethylammonium sulfate, 15 g of tripropylene glycol, and 54.9 g of water g, mixing and stirring at 45°C to prepare a mixed solution. Ultrasonic waves were irradiated to this mixed solution to emulsify and disperse all the monomers. Next, 0.15 g of azobis(isobutylamidine) dihydrochloride was added to the mixed solution, and 7 g of vinyl chloride was continuously pressed into the autoclave under a nitrogen atmosphere while the internal pressure of the autoclave was maintained at 0.3 MPa, and the temperature It was kept at 61°C and reacted for 6 hours to obtain an acrylic resin dispersion containing ...
Synthetic example 2~13
[0361] Except having used the material shown in Table 1 and Table 2, it carried out similarly to the synthesis example 1, and obtained the acrylic resin dispersion liquid.
[0362]
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com