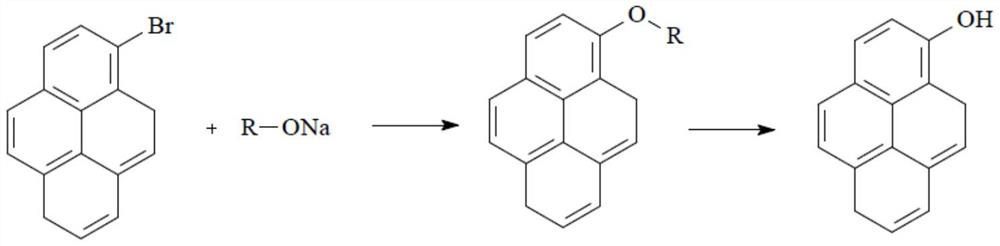
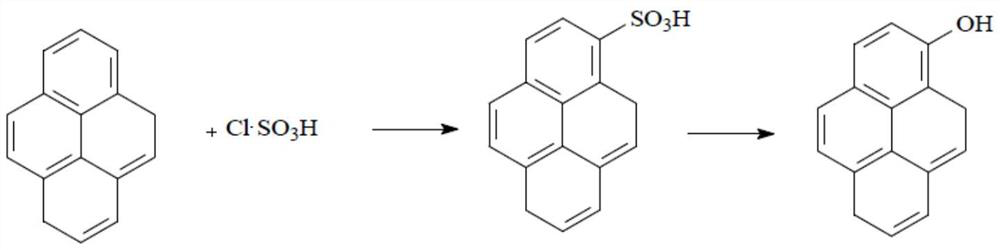
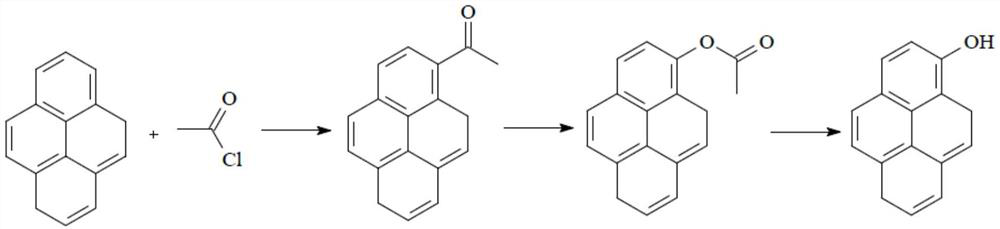
Preparation method of N-acylpyrene amine and preparation method of 1-hydroxypyrene
A technology of acylpyreneamine and hydroxypyrene, which is applied in the field of synthesis of 1-hydroxypyrene, and can solve problems such as harsh reaction conditions and cumbersome operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] In a typical embodiment of the present application, a preparation method of N-acylpyreneamine is provided, the preparation method comprising: adding C 2 ~C 10 The nitro compound containing α-H reacts with pyrene in a solid superacid and a first solvent to obtain N-acylpyreneamine.
[0027] This application ends with C 2 ~C 10 The nitro compound containing α-H and pyrene are the reaction raw materials, wherein C 2 ~C 10 The α-H of the nitro compound containing α-H itself has a strong acidity, and under the catalysis of a solid superacid, C 2 ~C 10 The α-H of the α-H-containing nitro compound is more easily pulled out, so that the C 2 ~C 10 The α-C of the nitro compound containing α-H is connected to the C on the 1-position of pyrene, and then through a one-step reaction to obtain N-acetylpyreneamine, and then the N-acetylpyreneamine obtained by this preparation method As a raw material to synthesize 1-hydroxypyrene. Compared with the traditional method—by perfor...
preparation Embodiment 1
[0043] First, add 20.2g (0.1mol) of pyrene, 8.25g (0.11mol) of nitroethane, and 80mL of acetic acid to a 250mL glass three-necked flask, and finally add 3g of HNF-5W perfluorosulfonic acid resin. Under nitrogen protection, the temperature is raised to Stir at 80°C for 8 hours, cool down to room temperature after passing the TLC control, and obtain N-acetylpyreneamine. MS(m / z)259[M] of N-acetylpyreneamine + , and the H NMR spectrum data are 1 H NMR (DMSO-d 6 )δ2.28 (s, 3H), 8.0-8.4 (m, 9H), 10.35 (s, 1H).
preparation Embodiment 2
[0045] The difference between the preparation example 2 of N-acylpyreneamine and the preparation example 1 of N-acylpyreneamine is that 20.2g (0.1mol) pyrene and 7.88g (0.105mol) nitroethane are added to finally obtain N-acetyl base pyrenamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com