Subunit vaccine against feline infectious peritonitis virus and application
A subunit vaccine, peritonitis virus technology, applied in vaccines, viruses, viral peptides, etc., can solve the problems of few drugs showing enough good therapeutic effect, and achieve the effects of easy expression and purification, high safety, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Construction of FIPV antigen (RBD-FTH1)
[0037] The schematic diagram of the fusion protein RBD-FTH1 structure is as follows: figure 1 shown. The specific construction method is as follows: add RBD-FTH1 to the start codon and stop codon, and insert the prokaryotic expression plasmid pBV220 ( Bam H I) Between restriction sites, plasmid mass spectrum such as figure 2 shown. DH5αcompetent cells were transformed with the recombinant plasmid, cultured overnight at 37°C, and positive clones were identified by screening and PCR. After being verified by sequencing, it is used for the expression of nanoantigen protein. The plasmid was transformed into expression strain BL21, and the expression of nanoantigen protein was induced at 42°C.
[0038] 2. Purification of RBD-HFn nanoantigens
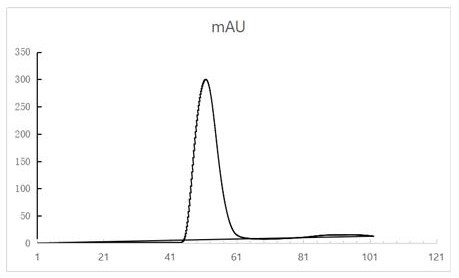
[0039] OD 280 =0.8 strains were sonicated and expressed in the supernatant after identification, and filtered through a 0.22 μm filter membrane to remove cell debris. After ultrafiltr...
Embodiment 2
[0041] Mouse Immunization Experiment
[0042] The RBD-FTH1 obtained in Example 1 was diluted to 100 μg / ml with physiological saline, and injected directly into mice. Then the 6-8 week-old Bal / C mice were immunized in groups. By intraperitoneal injection, each mouse was immunized three times on day 0, week 3 (day 21), and week 14 (day 108), with an inoculation volume of 100ul (10μg) each time. On the 10th, 31st, and 108th days, the mice were subjected to orbital blood collection. The mouse serum was left at room temperature for 2 hours, centrifuged at 3000 rpm for 15 minutes at 4°C, and immediately used in the FIPV neutralization assay.
[0043]
[0044] Neutralization experiment
[0045] Dilute the purified antibody 2-fold and mix with TCID 50 The final concentration of virus was mixed and incubated at 37°C for 1 hour. The mixture was added to a 96-well plate of Vero cells grown to about 80%. After 48 hours, the culture medium was discarded, and the cell lysate was wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com