High-activity Co-Ni-Fe co-inlaid non-noble metal catalyst as well as preparation method and application thereof
A non-precious metal and catalyst technology, applied in the field of highly active Co-Ni-Fe co-embedded non-precious metal catalyst and its preparation, can solve the problems of low catalyst activity and inability to construct a three-dimensional space structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The MOFs metal source ion Zn and Co, Ni, Fe atomic ratio is controlled at 4:4:3:1, wherein the concentration of MOFs metal source Zn ion is controlled at 4mol L -1 , the organic ligand concentration is 0.15mol L -1 : Preparation of mixed salt solution A: Dissolve 11.9g of zinc nitrate, 9.894g of cobalt sulfate, 8.7g of nickel nitrate, and 1.616g of ferrous acetate in 100mL of anhydrous methanol solution, and mix evenly by ultrasonic; dissolve 1.3g of dimethylimidazole in Obtain solution B in 100mL of anhydrous methanol solution; pour solution A into solution B, stir evenly, place in an oil bath at 100°C, and keep stirring for 12 hours. The reacted product was centrifuged and vacuum-dried at 90°C for 1 h, then placed in a tube furnace under an argon atmosphere at 1100°C for 0.5 h of pyrolysis, and the heating rate was controlled at 5°C min -1 . After pyrolysis, the target product Co-Ni-Fe catalyst is obtained.
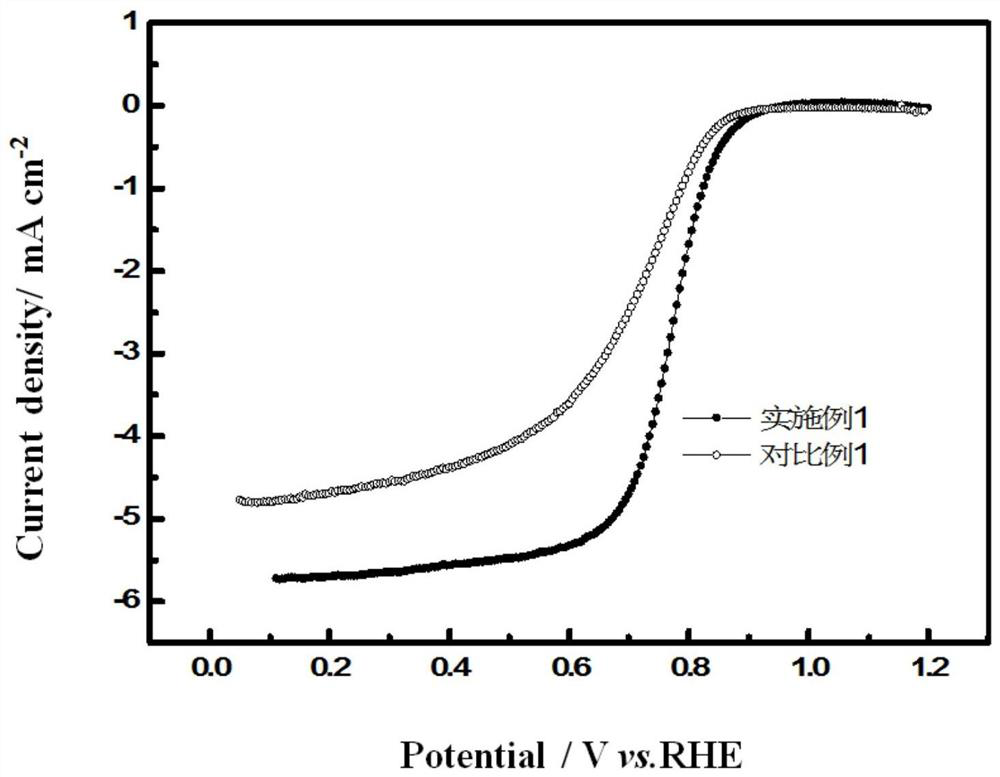
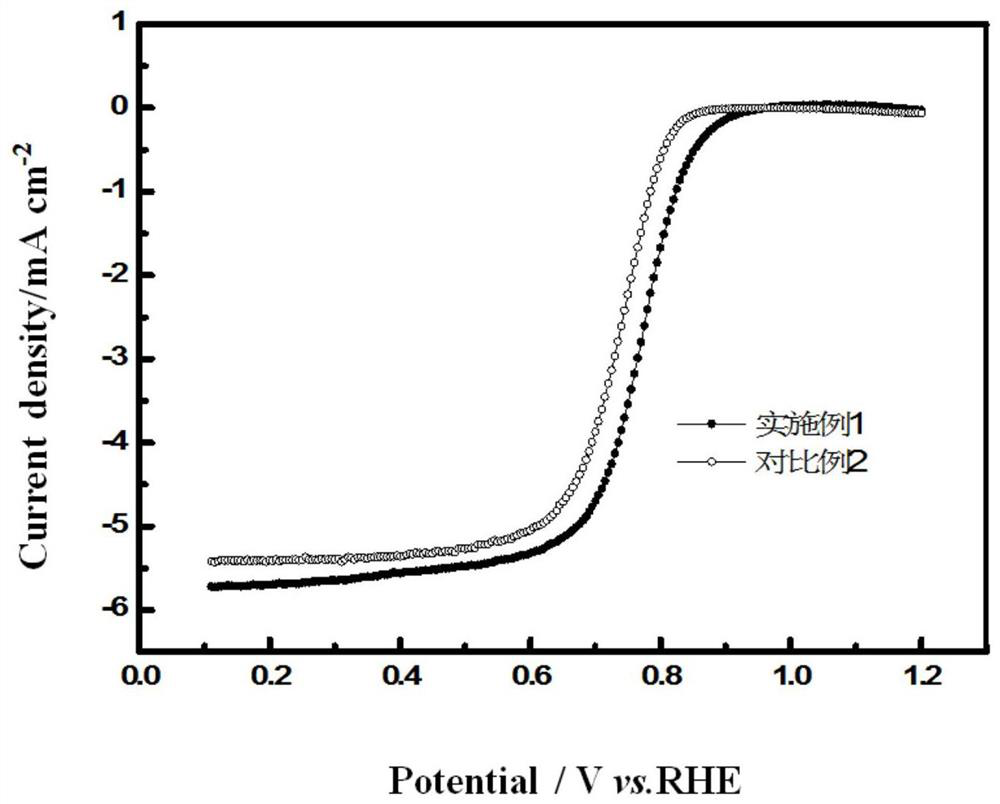
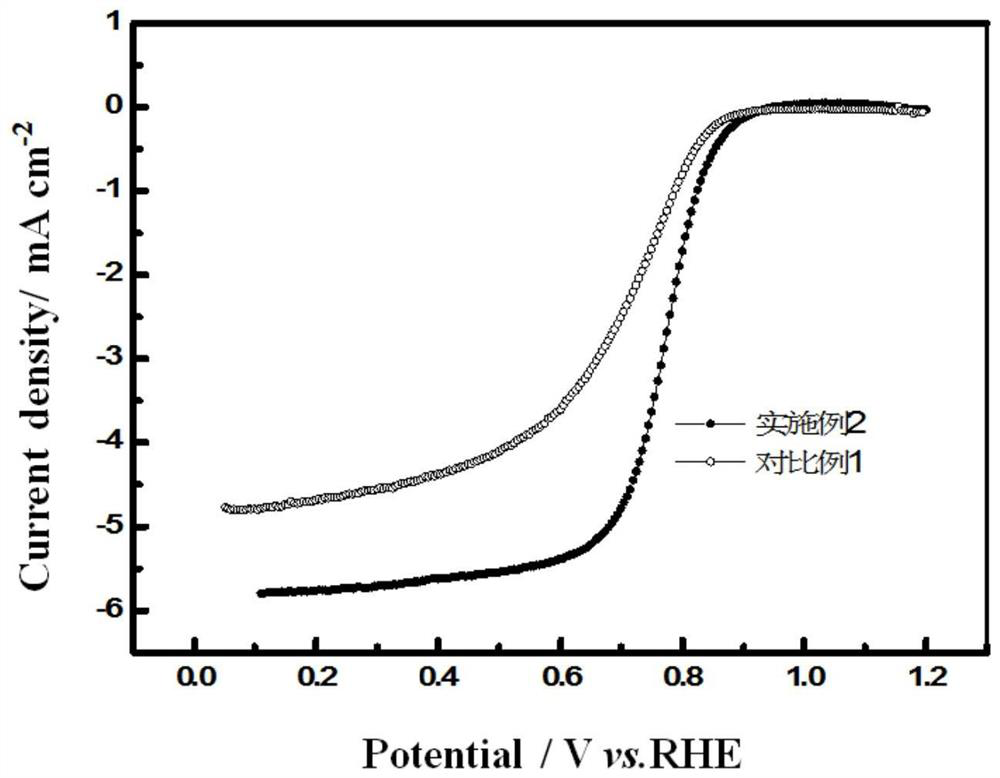
[0033] figure 1 It is a comparison chart of the perform...
Embodiment 2
[0036] The metal source ions and Co, Ni, Fe atomic ratio are controlled at 4:2:1:1, wherein the concentration of MOFs metal source ions is controlled at 4mol L -1 , the organic ligand concentration is 0.15mol L -1 : Prepare mixed salt solution A: Aluminum chloride 53.36g, cobalt chloride 5.8g, nickel chloride 2.97g, ferric chloride 4.04g are dissolved in 100mL DMF; 7.2g dihydroterephthalic acid is dissolved in 100mL The solution B was obtained in the DMF solution; the solution A was poured into the solution B, stirred evenly and placed in an oil bath at 20°C, and kept stirring for 48 hours. The reacted product was centrifuged and vacuum-dried at 60°C for 4 hours, then placed in a tube furnace for pyrolysis at 800°C for 3 hours under an argon atmosphere, and the heating rate was controlled at 2°C min -1 . After pyrolysis, the target product Co-Ni-Fe catalyst is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com