Preparation method of 1-chlorobutane
A technology of chlorobutane and n-butanol, which is applied in the field of preparation of 1-chlorobutane, can solve the problems of high equipment requirements, many by-products, and complex processes, and achieve the effects of good safety, less three wastes, and simple processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
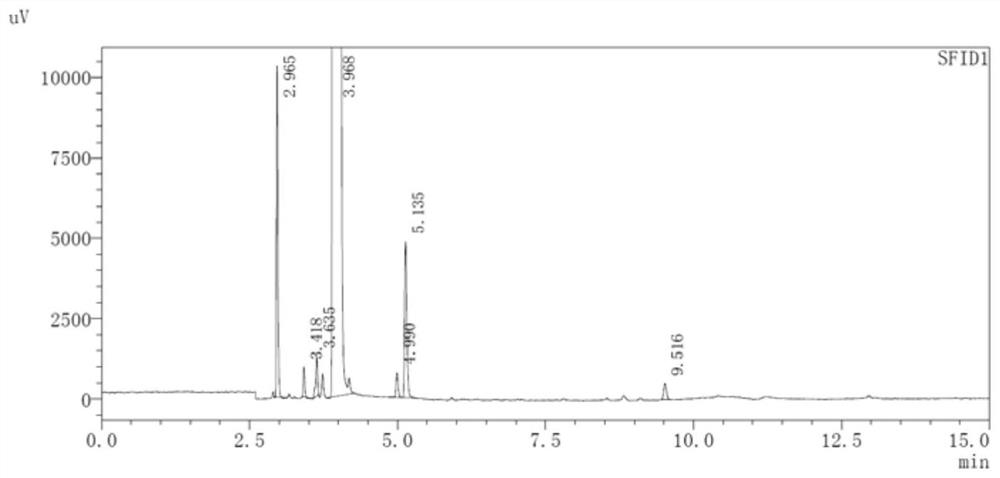
[0040] Add 200ml of 2,3,5,6-tetramethyldioxane, 20ml of purified water, and 200ml of n-butanol (2.19mol) into a 1000ml three-necked flask equipped with a stirrer and a thermometer, and stir to raise the temperature to 105°C , feed 199.8g (5.48mol) of hydrogen chloride gas, and distill while reacting. As the reaction time prolongs, fractions will slowly distill out gradually. After completing the heat preservation distillation and reacting for 2 hours, almost no further fractions will distill out, and the reaction is over. , after the obtained fractions were separated, the upper organic layer was 1-chlorobutane, and finally 195.4g (2.11mol) of 1-chlorobutane was obtained, the molar yield was 96.3%, and the GC purity was 99.83%. The chromatographic results are shown in figure 1 and Table 1. The 1-chlorobutane standard product is calibrated by GC, and the obtained product is 1-chlorobutane with a retention time of 3.968'; at the same time, n-butanol and dibutyl ether are calibrat...
Embodiment 2
[0046] Add 300ml of 2,3,5,6-tetramethyldioxane, 20ml of purified water, and 200ml of n-butanol (2.19mol) into a 1000ml three-necked flask equipped with a stirrer and a thermometer, and stir to raise the temperature to 115°C , feed 196.4g (5.38mol) of hydrogen chloride gas, and distill while reacting. As the reaction time prolongs, fractions will slowly distill out gradually. After completing the insulation distillation and reacting for 2 hours, almost no further fractions will distill out, and the reaction is over. After the obtained fractions were separated, the upper organic layer was 1-chlorobutane, and finally 195.9 g (2.12 mol) of 1-chlorobutane was obtained, with a molar yield of 96.6% and a GC purity of 99.79%.
Embodiment 3
[0048]Add 100ml of 2,3,5,6-tetramethyldioxane, 20ml of purified water, and 200ml of n-butanol (2.19mol) into a 1000ml three-necked flask equipped with a stirrer and a thermometer, and stir to raise the temperature to 85°C , feed 239.8g (6.57mol) of hydrogen chloride gas, and distill while reacting. As the reaction time prolongs, fractions will slowly distill out. After the obtained fractions were separated, the upper organic layer was 1-chlorobutane, and finally 194.6 g (2.11 mol) of 1-chlorobutane was obtained, with a molar yield of 96.0% and a GC purity of 99.87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com