Novel application of N-(2-phenylethyl)-5-phenyl-pyridine-2-formamide and medicine thereof
A technology of phenylethyl and formamide, applied in the field of medicine, can solve the problems of aggravating acute lung injury, edema, and increased permeability of pulmonary vascular endothelial cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
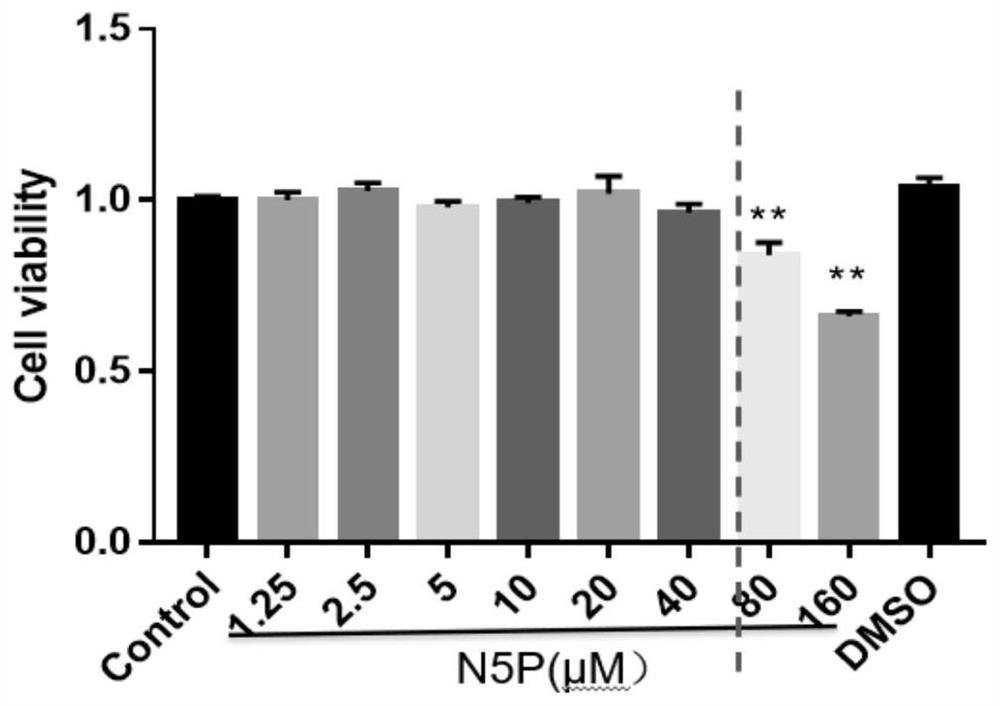
[0077] The test of compound N-(2-phenylethyl)-5-phenyl-pyridine-2-carboxamide on LPS-induced RAW264.7 macrophages:
[0078] (1) MTT method: RAW264.7 macrophages were seeded in a 96-well plate at a seeding density of about 4000 cells / well. Cultivate for 24 hours, add different concentrations of compound N-(2-phenylethyl)-5-phenyl-pyridine-2-carboxamide and solvent control DMSO in groups, respectively control group (Control), drug group with different concentrations (compound The concentration of N-(2-phenylethyl)-5-phenyl-pyridine-2-carboxamide is 1.25 μM, 2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM), DMSO solvent control group. Continue to cultivate for 24h. Add 5g·L to each well after culturing -1 20 μL of MTT solution and continue to incubate for 4 h. Aspirate the medium, add 150 μL of DMSO to each well, shake and mix well. Measure the OD value of each well at 492nm with a microplate reader, and record the results. Cell viability was plotted against dose. Result c...
Embodiment 2
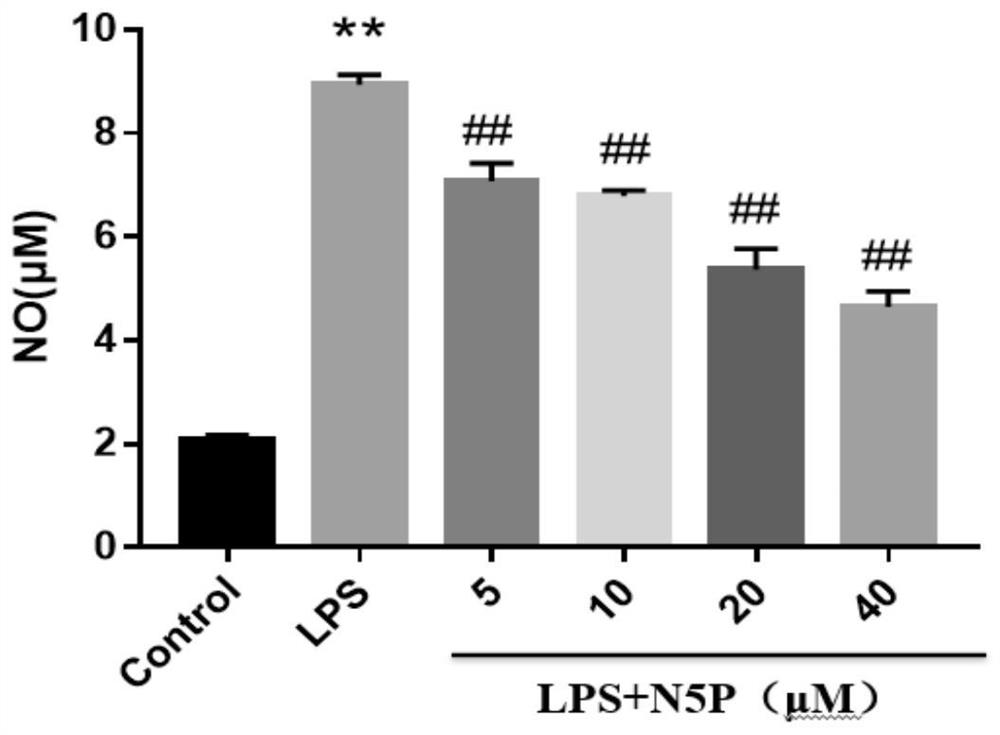
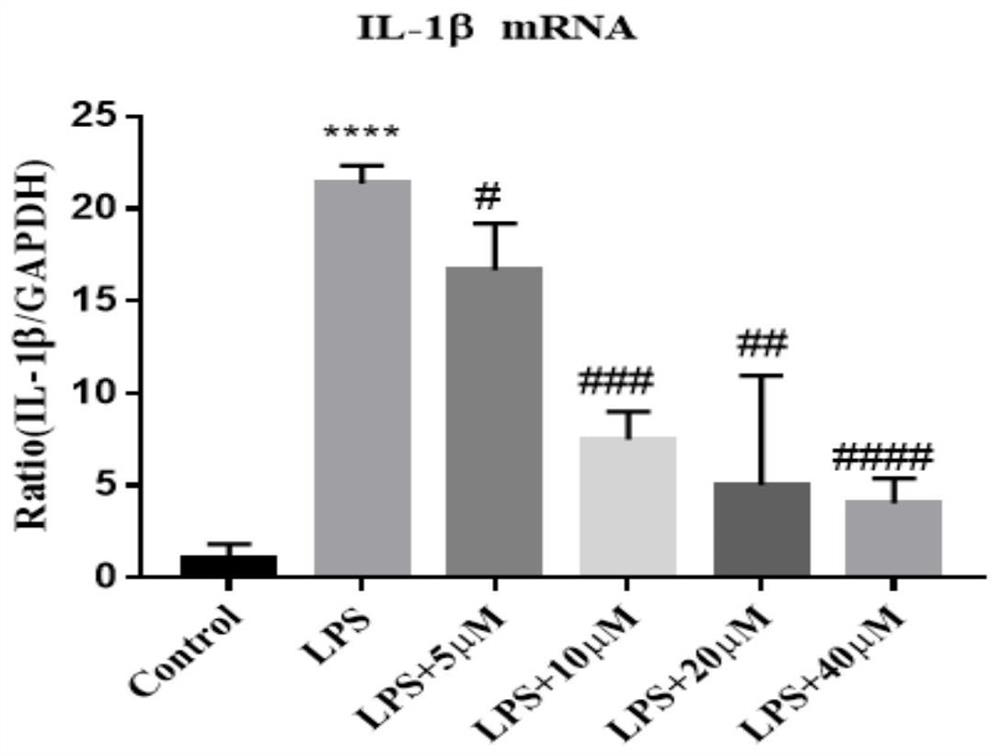
[0087] Compound N-(2-phenylethyl)-5-phenyl-pyridine-2-carboxamide was tested on animal model of acute lung injury:
[0088] Male SD rats weighing 200g-250g were adaptively cultured for 1-2 days, and divided into normal group (Control), model group (LPS5mg / kg), low-dose group (LPS5mg / kg+25mg / kg), middle-dose group (LPS5mg / kg+50mg / kg), high-dose group (LPS5mg / kg+100mg / kg), positive drug control group (LPS5mg / kg+2mg / kgYC-1) 6-10 in each group. The SD rats were intraperitoneally injected with the above-mentioned doses of drugs for 3 days, and the normal group was injected with the same volume of solvent as control. On the 4th day, the model was established by instilling LPS into the trachea. To establish the ALI model, the rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg / kg), then intratracheal injection of LPS (5 mg / kg, 200 ul), and the rats in the control group were given 200 ul of normal saline in the trachea. Rats were sacrificed twenty-four...
Embodiment 3
[0091] The obtained total inflammatory cell mass was resuspended with incomplete medium RPMI1640 by pipetting, divided into 15ml centrifuge tubes, and centrifuged at 1500rpm×10min, and the supernatant was discarded. Count cells according to 5 x 10 5 Inoculate 6-well plates. The cells were suspended in RPMI1640 medium with 10% FBS, and cultured in an incubator for 2 hours. Aspirate the cell suspension, wash with RPMI1640 medium, remove unattached cells, and add complete medium RPMI1640. After thorough mixing, incubate for 24 hours in a cell culture incubator at 37°C, 5% CO2, and saturated humidity to make the macrophages adhere to the wall. Shake the flask to suspend non-adherent cells and aspirate the cell suspension. Wash with PBS three times to remove unattached cells. The macrophages were washed three times with RPMI1640 culture medium, and the cells were suspended in RPMI1640 medium containing 10% FBS to obtain purified primary alveolar macrophages. Cell identificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com