Detection method for in-vitro inhibition of Th1 and Th17 by mesenchymal stem cells
A mesenchymal stem cell and detection method technology, applied in the field of stem cell biology, can solve the problems of not having a good mesenchymal stem cell immune regulation ability, ineffective effect, and poor repeatability, so as to improve repeatability, effect, and The effect of expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
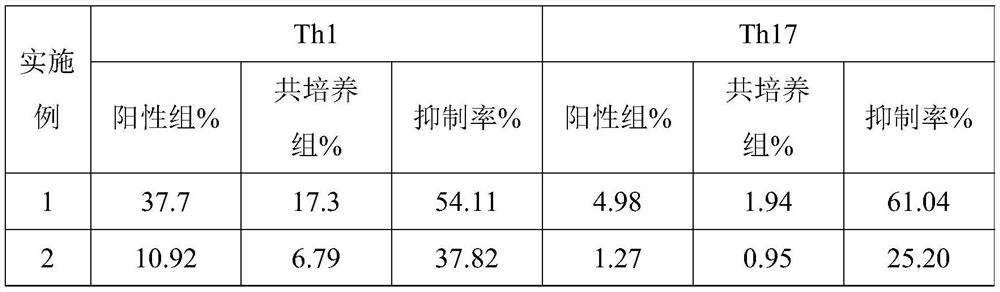
Embodiment 1
[0074] This example provides a detection method for mesenchymal stem cells to inhibit Th1 and Th17 in vitro, including:
[0075] (1) MSC cell inoculation: take the MSC cell suspension, centrifuge at 400g for 5min, remove the supernatant, add complete medium to resuspend, sample and count; according to the counting results, adjust the cell concentration to 2×10 5 / mL; inoculate the adjusted cell suspension in 24-well culture plate, 500 μl per well, and inoculate 2 wells in total. Place the inoculated cell plates in a carbon dioxide incubator (CO 2 Concentration setting: 5.0%, temperature setting: 37.0°C), culture for 20-24 hours;
[0076] (2) MSC deproliferation treatment: After MSC cells were cultured for 20-24 hours, observe the cell adhesion under the microscope; discard the medium in each well, add 500 μl of complete medium containing 10 μg / mL mitomycin C to each well, placed in a carbon dioxide incubator (CO 2 Concentration setting: 5.0%, temperature setting: 37.0°C), i...
Embodiment 2
[0084] This example provides a detection method for mesenchymal stem cells to inhibit Th1 and Th17 in vitro. Centrifuge to get the cell pellet, add medium containing 1μg / mL PHA to stimulate the cells for 1h, then add 10μg / mL BFA, put in CO 2 Continue to incubate in the incubator for 5 hours; blow and disperse the aggregated cells evenly every 2 hours; centrifuge at 500g for 5 minutes, wash the obtained cell pellet twice with 2 mL of PBS, and then add 100 μL of PBS to resuspend to obtain a resuspension solution.
Embodiment 3
[0086]This example provides a detection method for mesenchymal stem cells inhibiting Th1 and Th17 in vitro. The specific implementation method is the same as in Example 1, except that in the step (5), 100 μL of fixative and 100 μL of fixative and After vortexing 100 μL of membrane disrupting agent for 5 seconds and fixing for 15 minutes, add staining antibody, incubate in the dark for 20 minutes, add 3 mL of balanced salt solution, centrifuge at 400 g for 5 minutes, and add 300 μL of resuspension solution to the obtained cell pellet for detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com