Human phosphorylated vasodilatation stimulating phosphoprotein magnetic particle chemiluminiscence immunoassay quantitative detection kit and detection method and application thereof
A technology of chemiluminescence immunity and vasodilation, which is applied in the direction of chemiluminescence/bioluminescence, analysis by causing chemical reactions of materials, and measurement devices. It can solve problems such as narrow linear range, poor stability, and low sensitivity, and achieve low price. , wide linearity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the preparation of kit
[0038] The kit of the present invention uses magnetic microparticles as the solid phase of the immune reaction, uses a chemiluminescence immunoassay method in cooperation with a chemiluminescence measuring instrument, and is used for measuring the content of human phosphorylated vasodilator-stimulated phosphoprotein in human body samples. The technical principle of the reaction is: sample to be tested, calibrator and alkaline phosphatase-labeled human phosphorylated vasodilator-stimulated phosphoprotein monoclonal antibody enzyme conjugate, magnetic bead-coated human phosphorylated vasodilator-stimulated phosphoprotein monoclonal antibody The coating is combined to form a VASP protein monoclonal antibody enzyme conjugate-human phosphorylated vasodilator-stimulated phosphoprotein-VASP protein monoclonal antibody coating complex, which is directly precipitated in an external magnetic field, and the complex formed by the immune reactio...
Embodiment 2
[0061] Embodiment 2, the test method of kit
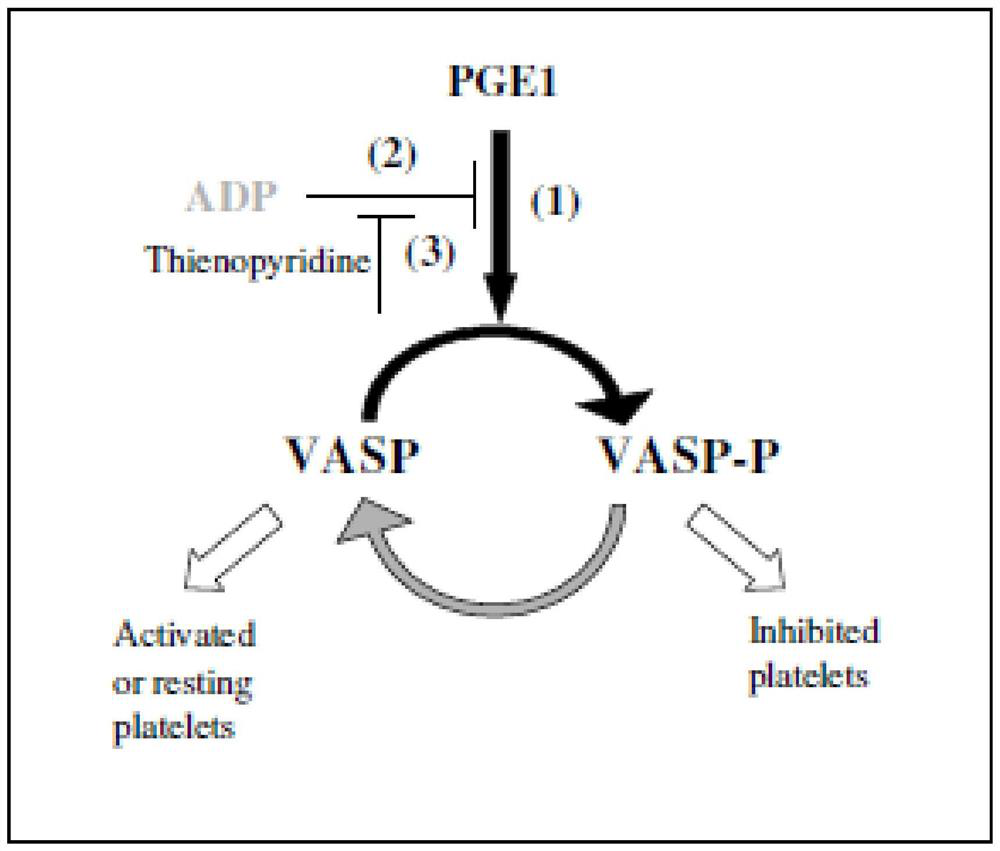
[0062] (1) Sample addition and incubation: pipette 50 μL of human phosphorylated vasodilator-stimulated phosphoprotein calibrator or fresh patient sample into the reaction tube, add 50 μL of PGE1 lyser 1 and PGE1+ADP lyser 2, lyser 1 and lyser respectively 2 The working principle diagram is as follows: figure 1 As indicated, incubate at 37°C for about 10 minutes; then add alkaline phosphatase-labeled human phosphorylated vasodilator-stimulated phosphoprotein monoclonal antibody enzyme conjugate 50 μL and magnetic bead-coated human phosphorylated vasodilator-stimulated phosphoprotein monoclonal Antibody coating 50μL, incubate at 37℃ for about 10 minutes;
[0063] (2) Magnetic separation cleaning: put the reaction tube after the incubation reaction on the magnetic separation rack and let it stand for 1 minute, remove the supernatant; add 300 μL of magnetic bead coating buffer for the first time, and place it on the magnetic separati...
Embodiment 3
[0065] Embodiment 3, the performance test result of kit
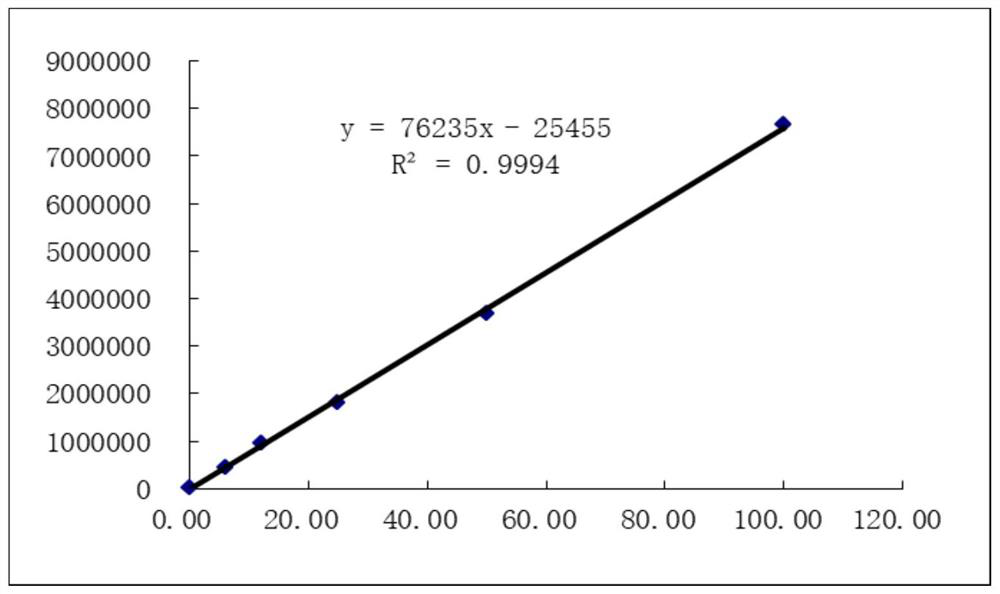
[0066] Kit performance evaluation index: The accuracy, linearity, precision, specificity and stability of the kit prepared by this method were measured. Draw the standard curve after using the protein calibrator to detect, the standard curve is as follows figure 2 As shown, the standard curve formula y=76235x–25455, R 2 =0.9994, and evaluate the linearity of the test results at the same time, the test results are shown in the table below:
[0067] Concentrationng / mL Luminous value 1 Luminous value 2 average 0.000 9985 9847 9916 6.125 464998 447115 456057 12.250 980998 953436 967217 25.000 1890029 1741507 1815768 50.000 3777745 3586306 3682026 100.000 7751427 7565142 7658285
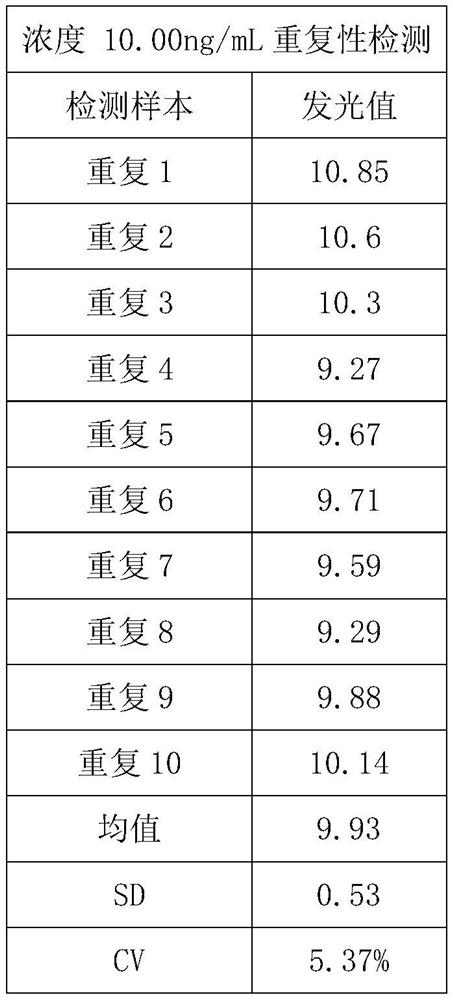
[0068] The repeatability test was performed with a protein calibrator with a concentration of 10ng / ml, and the test results are shown in the table below:
[0069]
[0070] From th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com