Antibacterial peptide liquid composition and preparation thereof
A liquid composition, antibacterial peptide technology, applied in the direction of antibacterial drugs, drug combination, drug delivery, etc., to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
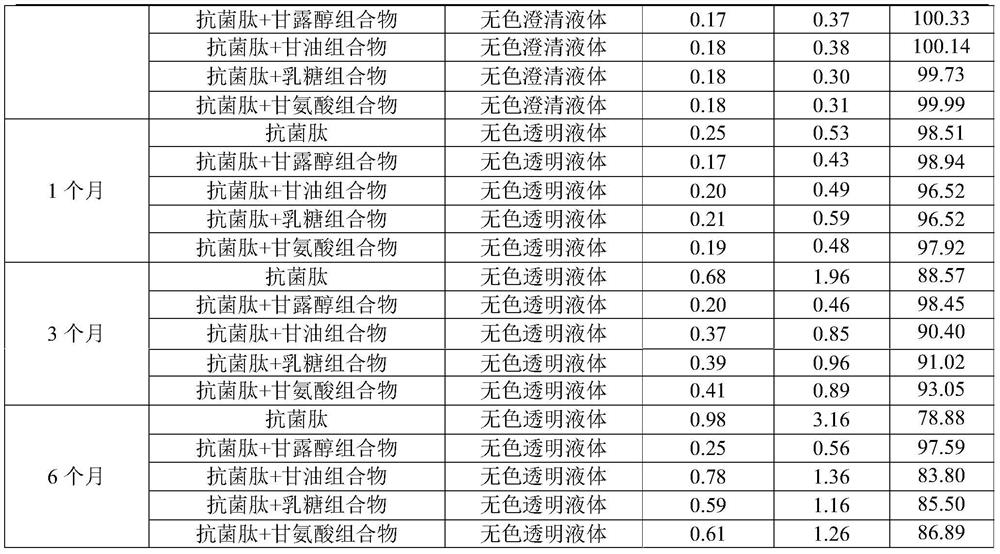
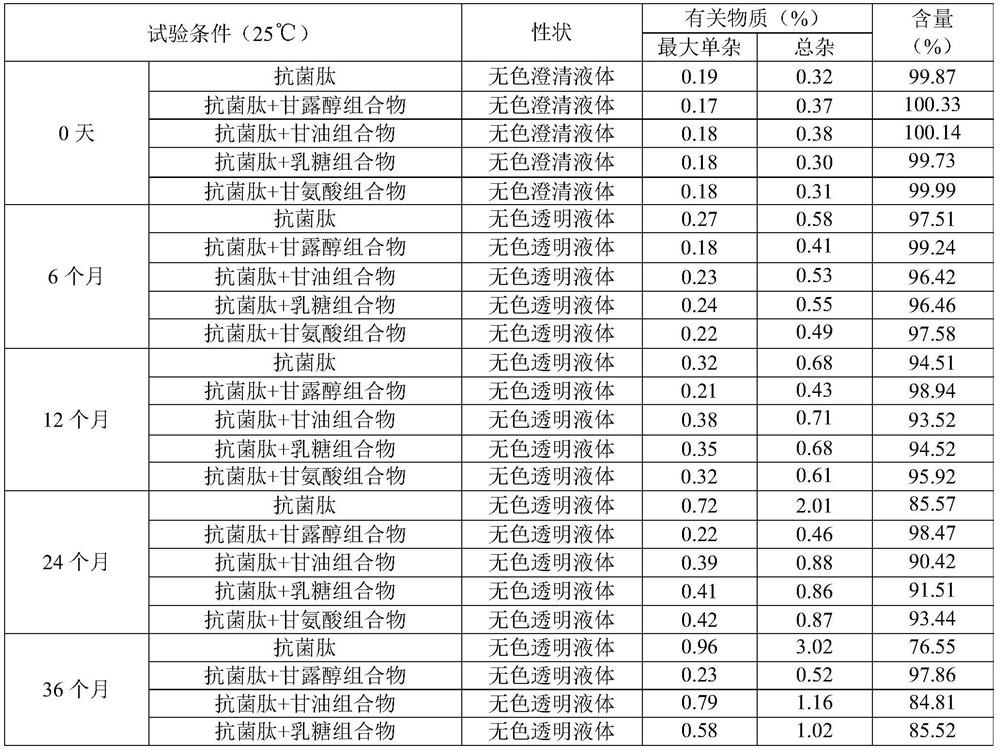
[0048] Embodiment 1, antimicrobial peptide composition stabilizer screening
[0049] Select mannitol, glycerol, lactose and glycine etc. as the stabilizing agent of antimicrobial peptide, prepare respectively the aqueous solution of antimicrobial peptide composition (antimicrobial peptide concentration is 2 ‰), investigate the stability of antimicrobial peptide composition, detection index comprises character, related substance ( The largest single impurity and total impurity), content, etc.
[0050] Related substance testing methods:
[0051] According to the high-performance liquid chromatography (Chinese Pharmacopoeia 2015 Edition, Appendix V D), using octadecylsilane-bonded silica gel as a filler, take 20 μL of the solution and inject it into a liquid chromatograph, and record the chromatogram. Relevant substances and limits: known impurities A, B, C, D and unknown single impurities, each impurity shall not exceed 1.0%, and the maximum simple impurities shall be summarize...
Embodiment 2
[0067] The molar concentration investigation of embodiment 2 antimicrobial peptide composition buffer system and buffer system
[0068] The antibacterial peptide composition buffer system is investigated according to the combination of the following table, and the mass concentration of the antimicrobial peptide in each antimicrobial peptide composition buffer system in the following table is 4‰.
[0069] Table 4 antimicrobial peptide composition buffer system
[0070]
[0071]
[0072] Table 5 antimicrobial peptide composition buffer system test investigation result
[0073]
[0074] It can be seen from Table 5 that the selection of disodium hydrogen phosphate and citric acid buffer system can ensure that the antimicrobial peptide composition solution can still have more than 98% of the antimicrobial peptide content in 3 months (at 25° C.). Therefore, disodium hydrogen phosphate and citric acid buffer system is the preferred buffer system.
[0075] For the preferred...
Embodiment 3
[0079] Embodiment 3, pH value investigation of antimicrobial peptide composition
[0080] Select mannitol (1%) as the stability, disodium hydrogen phosphate and citric acid as the buffer system (0.015M) to prepare different pH values (3.0, 3.5, 4.5, 5.5, 6.5) antimicrobial peptide (2‰) composition solution , to investigate the stability of the antimicrobial peptide composition under different pH conditions, and the results are shown in Table 7.
[0081] Select hydrochloric acid simultaneously as pH regulator, adjust the pH value of antimicrobial peptide aqueous solution to 4.5, investigate the stability of antimicrobial peptide, and compare with disodium hydrogen phosphate and citric acid as the antimicrobial peptide composition solution of buffer system, the results are shown in the table 8.
[0082] The influence of table 7 composition pH value on antimicrobial peptide stability
[0083]
[0084] The influence (pH4.5) of different pH regulators of table 8 on the stabi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com