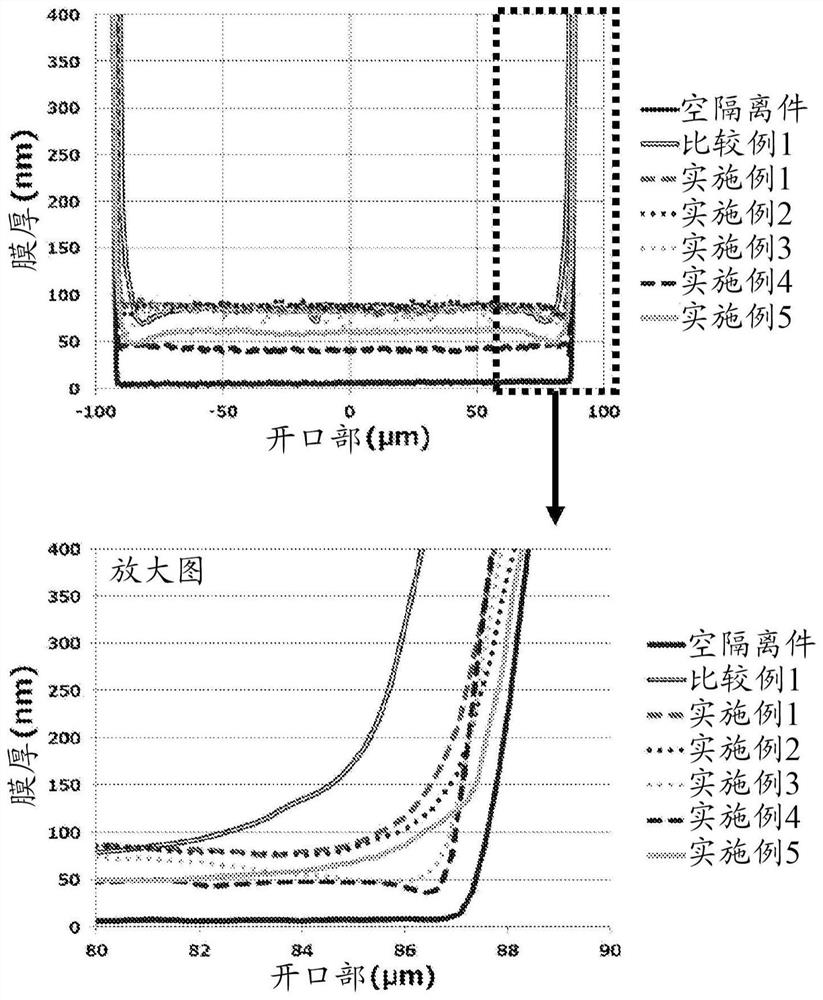
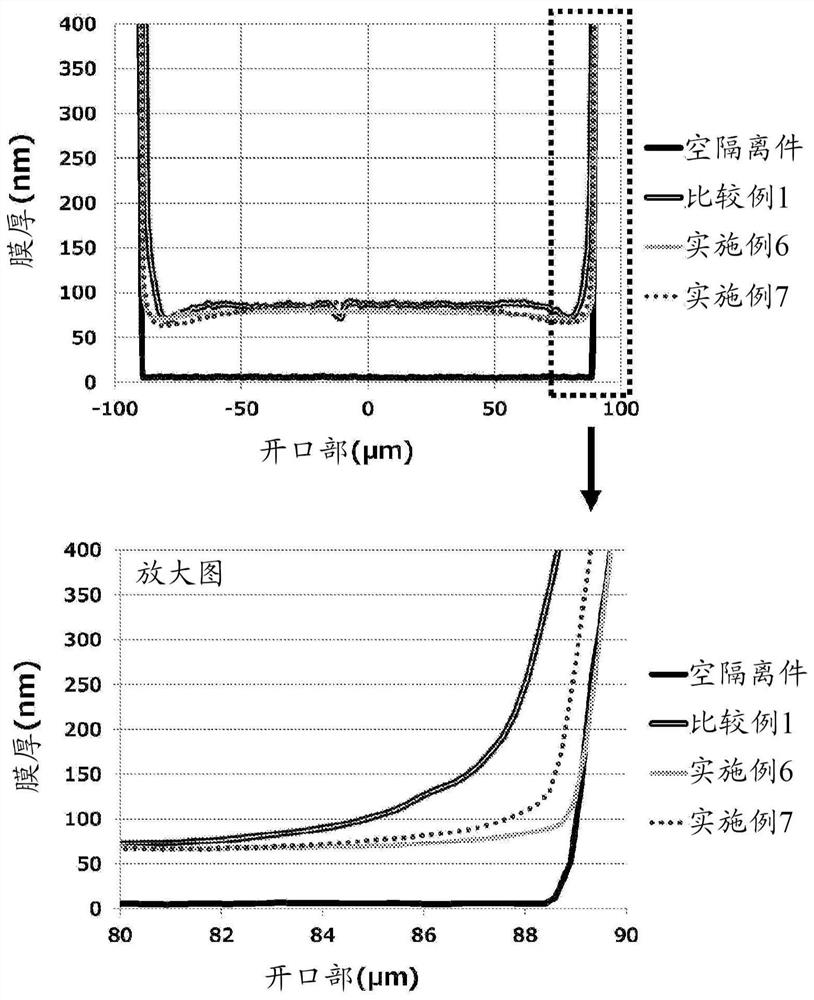
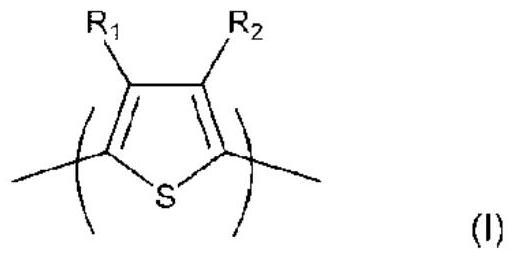
Ink composition
A composition and ink technology, applied in the direction of ink, application, electrical components, etc., can solve the problem of not grasping the way of accumulation phenomenon of ink composition, and achieve the effect of inhibiting accumulation phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0485] The meanings of the abbreviations used in the following examples are as follows.
[0486] MMA: methyl methacrylate
[0487] HEMA: 2-Hydroxyethyl methacrylate
[0488] HPMA: 4-Hydroxyphenyl methacrylate
[0489] HPMA-QD: A compound synthesized by the condensation reaction of 1 mol of 4-hydroxyphenyl methacrylate and 1.1 mol of 1,2-naphthoquinone-2-diazido-5-sulfonyl chloride
[0490] CHMI: N-Cyclohexylmaleimide
[0491] PFHMA: 2-(perfluorohexyl)ethyl methacrylate
[0492] MAA: methacrylic acid
[0493] AIBN: α,α'-Azobisisobutyronitrile
[0494] QD1: Pass 1mol of α,α,α'-tris(4-hydroxyphenyl)-1-ethyl-4-isopropylbenzene and 1.5mol of 1,2-naphthoquinone-2-diazide-5 Compounds synthesized by the condensation reaction of sulfonyl chloride
[0495] GT-401: Butanetetracarboxylic acid tetrakis(3,4-epoxycyclohexylmethyl)-modified ε-caprolactone (trade name: EPOLEADGT-401 (manufactured by Daicel Corporation))
[0496] PGME: Propylene Glycol Monomethyl Ether
[0497] PGMEA: ...
manufacture example 1
[0508] Preparation of S-poly(3-MEET)amine adduct
[0509] 500 g of an aqueous dispersion of S-poly(3-MEET) (0.598% solids in water) was mixed with 0.858 g of triethylamine, and the resulting mixture was evaporated by rotary evaporation to dry and solidify. The resulting residue was then further dried overnight in a vacuum oven at 50° C. to obtain 3.8 g of S-poly(3-MEET)amine adduct as a black powder product.
manufacture example 2
[0511] 2.00 g of the S-poly(3-MEET) amine adduct obtained in Production Example 1 was dissolved in 100 mL of 28% ammonia water (manufactured by Junsei Chemical Co., Ltd.), and the resulting solution was stirred overnight at room temperature. The obtained reaction mixture was subjected to reprecipitation treatment with 1500 mL of acetone, and the precipitate was recovered by filtration. The obtained precipitate was dissolved again in 20 mL of water and 7.59 g of triethylamine (manufactured by Tokyo Chemical Industry Co., Ltd.), and stirred at 60° C. for 1 hour. After cooling the obtained reaction mixture, reprecipitation treatment was performed with a mixed solvent of 1000 mL of isopropanol and 500 mL of acetone, and the precipitate was collected by filtration. The obtained precipitate was vacuum-dried at 0 mmHg and 50° C. for 1 hour to obtain 1.30 g of S-poly(3-MEET)-A as a charge-transporting substance treated with ammonia water.
[0512] [2] Preparation of charge transport ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com